
Meconium aspiration syndrome (MAS) also known as neonatal aspiration of meconium is a medical condition affecting newborn infants. It describes the spectrum of disorders and pathophysiology of newborns born in meconium-stained amniotic fluid (MSAF) and have meconium within their lungs. Therefore, MAS has a wide range of severity depending on what conditions and complications develop after parturition. Furthermore, the pathophysiology of MAS is multifactorial and extremely complex which is why it is the leading cause of morbidity and mortality in term infants.

A pulmonary alveolus, also known as an air sac or air space, is one of millions of hollow, distensible cup-shaped cavities in the lungs where pulmonary gas exchange takes place. Oxygen is exchanged for carbon dioxide at the blood–air barrier between the alveolar air and the pulmonary capillary. Alveoli make up the functional tissue of the mammalian lungs known as the lung parenchyma, which takes up 90 percent of the total lung volume.

Infantile respiratory distress syndrome (IRDS), also called respiratory distress syndrome of newborn, or increasingly surfactant deficiency disorder (SDD), and previously called hyaline membrane disease (HMD), is a syndrome in premature infants caused by developmental insufficiency of pulmonary surfactant production and structural immaturity in the lungs. It can also be a consequence of neonatal infection and can result from a genetic problem with the production of surfactant-associated proteins.

Liquid breathing is a form of respiration in which a normally air-breathing organism breathes an oxygen-rich liquid (such as a perfluorocarbon), rather than breathing air, by selecting a liquid that can hold a large amount of oxygen and is capable of CO2 gas exchange.
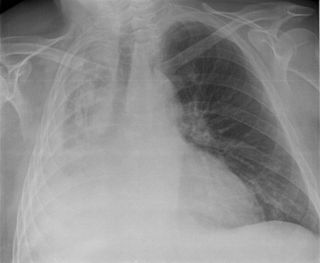
Atelectasis is the collapse or closure of a lung resulting in reduced or absent gas exchange. It is usually unilateral, affecting part or all of one lung. It is a condition where the alveoli are deflated down to little or no volume, as distinct from pulmonary consolidation, in which they are filled with liquid. It is often called a collapsed lung, although that term may also refer to pneumothorax.
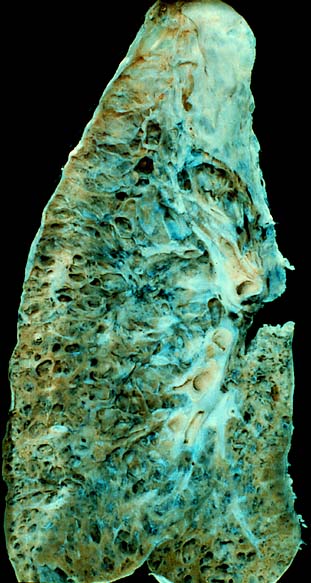
Interstitial lung disease (ILD), or diffuse parenchymal lung disease (DPLD), is a group of respiratory diseases affecting the interstitium and space around the alveoli of the lungs. It concerns alveolar epithelium, pulmonary capillary endothelium, basement membrane, and perivascular and perilymphatic tissues. It may occur when an injury to the lungs triggers an abnormal healing response. Ordinarily, the body generates just the right amount of tissue to repair damage, but in interstitial lung disease, the repair process is disrupted, and the tissue around the air sacs (alveoli) becomes scarred and thickened. This makes it more difficult for oxygen to pass into the bloodstream. The disease presents itself with the following symptoms: shortness of breath, nonproductive coughing, fatigue, and weight loss, which tend to develop slowly, over several months. The average rate of survival for someone with this disease is between three and five years. The term ILD is used to distinguish these diseases from obstructive airways diseases.

Pulmonary surfactant is a surface-active complex of phospholipids and proteins formed by type II alveolar cells. The proteins and lipids that make up the surfactant have both hydrophilic and hydrophobic regions. By adsorbing to the air-water interface of alveoli, with hydrophilic head groups in the water and the hydrophobic tails facing towards the air, the main lipid component of surfactant, dipalmitoylphosphatidylcholine (DPPC), reduces surface tension.
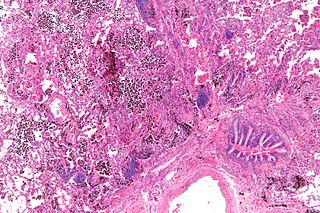
Pulmonary hemorrhage is an acute bleeding from the lung, from the upper respiratory tract and the trachea, and the pulmonary alveoli. When evident clinically, the condition is usually massive. The onset of pulmonary hemorrhage is characterized by a cough productive of blood (hemoptysis) and worsening of oxygenation leading to cyanosis. Treatment should be immediate and should include tracheal suction, oxygen, positive pressure ventilation, and correction of underlying abnormalities such as disorders of coagulation. A blood transfusion may be necessary.
Antenatal steroids, also known as antenatal corticosteroids, are medications administered to pregnant women expecting a preterm birth. When administered, these steroids accelerate the maturation of the fetus' lungs, which reduces the likelihood of infant respiratory distress syndrome and infant mortality. The effectiveness of this corticosteroid treatment on humans was first demonstrated in 1972 by Sir Graham Liggins and Ross Howie, during a randomized control trial using betamethasone.

Respiratory diseases, or lung diseases, are pathological conditions affecting the organs and tissues that make gas exchange difficult in air-breathing animals. They include conditions of the respiratory tract including the trachea, bronchi, bronchioles, alveoli, pleurae, pleural cavity, the nerves and muscles of respiration. Respiratory diseases range from mild and self-limiting, such as the common cold, influenza, and pharyngitis to life-threatening diseases such as bacterial pneumonia, pulmonary embolism, tuberculosis, acute asthma, lung cancer, and severe acute respiratory syndromes, such as COVID-19. Respiratory diseases can be classified in many different ways, including by the organ or tissue involved, by the type and pattern of associated signs and symptoms, or by the cause of the disease.
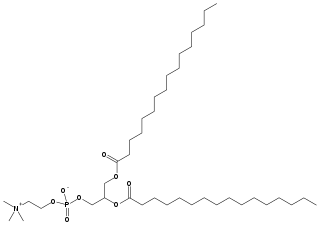
Dipalmitoylphosphatidylcholine (DPPC) is a phospholipid (and a lecithin) consisting of two C16 palmitic acid groups attached to a phosphatidylcholine head-group.

Colfosceril palmitate is a drug used as a pulmonary surfactant. It is a drug that is used in surfactant deficient conditions such as infant respiratory distress syndrome in newborns.

The lecithin–sphingomyelin ratio is a test of fetal amniotic fluid to assess for fetal lung immaturity. Lungs require surfactant, a soap-like substance, to lower the surface pressure of the alveoli in the lungs. This is especially important for premature babies trying to expand their lungs after birth. Surfactant is a mixture of lipids, proteins, and glycoproteins, lecithin and sphingomyelin being two of them. Lecithin makes the surfactant mixture more effective.

Diffuse alveolar damage (DAD) is a histologic term used to describe specific changes that occur to the structure of the lungs during injury or disease. Most often DAD is described in association with the early stages of acute respiratory distress syndrome (ARDS). It is important to note that DAD can be seen in situations other than ARDS (such as acute interstitial pneumonia) and that ARDS can occur without DAD.
Lucinactant is a liquid medication used to treat infant respiratory distress syndrome. It is a pulmonary surfactant for infants who lack enough natural surfactant in their lungs. Whereas earlier medicines of the class, such as beractant, calfactant (Infasurf), and poractant (Curosurf), are derived from animals, lucinactant is synthetic. It was approved for use in the United States by the U.S. Food and Drug Administration (FDA) on March 6, 2012.
Poractant alfa is a pulmonary surfactant sold under the brand name Curosurf by Chiesi Farmaceutici. Poractant alfa is an extract of natural porcine lung surfactant. As with other surfactants, marked improvement on oxygenation may occur within minutes of the administration of poractant alfa. The new generic form of surfactant is Varasurf developed in PersisGen Co. and commercialized by ArnaGen Pharmad. It has fully comparable quality profile with Curosurf.
Calfactant, also known as Infasurf, is an intratracheal suspension derived from the natural surfactant in calf lungs. It is used in premature infants with lung surfactant deficiency that causes infant respiratory distress syndrome (IRDS).

Henrik Verder is a pediatrician and the inventor of the INSURE and LISA methods combined with nasal CPAP. In 1989 he used this pioneering method to successfully treat the first premature infant with severe RDS. Verder is a significant researcher within the field of paediatrics, with more than 50 publications and over 500 citations.

Pulmonary surfactant is used as a medication to treat and prevent respiratory distress syndrome in newborn babies.

Henry Lewis Halliday was a British-Irish peaditrician and neonatologist. In 2021, Halliday was awarded the James Spence Medal for research into neonatology, for coordinating two of the largest neonatal multicentre trials for prevention and treatment of a number of neonatal respiratory illnesses and for a breakthrough in the development of a new lung surfactant that brought relief to very small babies suffering from infant respiratory distress syndrome (RDS).















