
Leukemia is a group of blood cancers that usually begin in the bone marrow and result in high numbers of abnormal blood cells. These blood cells are not fully developed and are called blasts or leukemia cells. Symptoms may include bleeding and bruising, bone pain, fatigue, fever, and an increased risk of infections. These symptoms occur due to a lack of normal blood cells. Diagnosis is typically made by blood tests or bone marrow biopsy.
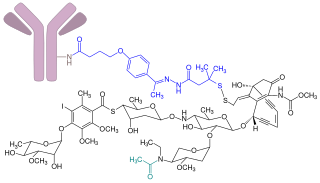
Gemtuzumab ozogamicin, sold under the brand name Mylotarg, is an antibody-drug conjugate that is used to treat acute myeloid leukemia.

Acute myeloid leukemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood and interfere with normal blood cell production. Symptoms may include feeling tired, shortness of breath, easy bruising and bleeding, and increased risk of infection. Occasionally, spread may occur to the brain, skin, or gums. As an acute leukemia, AML progresses rapidly, and is typically fatal within weeks or months if left untreated.

Acute myeloblastic leukemia with maturation (M2) is a subtype of acute myeloid leukemia (AML).

Cluster of differentiation antigen 135 (CD135) also known as fms like tyrosine kinase 3, receptor-type tyrosine-protein kinase FLT3, or fetal liver kinase-2 (Flk2) is a protein that in humans is encoded by the FLT3 gene. FLT3 is a cytokine receptor which belongs to the receptor tyrosine kinase class III. CD135 is the receptor for the cytokine Flt3 ligand (FLT3L).
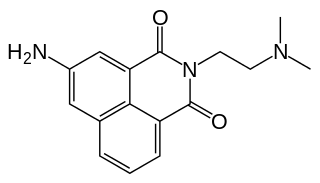
Amonafide was a drug that was being studied in the treatment of cancer. It belongs to a novel family of chemotherapeutic drugs called Naphthalimides and is a potential topoisomerase inhibitor and DNA intercalator.

Lestaurtinib is a tyrosine kinase inhibitor structurally related to staurosporine. This semisynthetic derivative of the indolocarbazole K252a was investigated by Cephalon as a treatment for various types of cancer. It is an inhibitor of the kinases fms-like tyrosine kinase 3 (FLT3), Janus kinase 2 (JAK2), tropomyosin receptor kinase (trk) A (TrkA), TrkB and TrkC.

Laniquidar (INN) is a third generation P-glycoprotein inhibitor that underwent clinical studies for acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). It has been discontinued because of its low bioavailability and a high variability with how the patients responded to the drug.

Sapacitabine is a chemotherapeutic drug developed by US biotechnology firm Cyclacel currently undergoing clinical trials against leukemia.

Childhood leukemia is leukemia that occurs in a child and is a type of childhood cancer. Childhood leukemia is the most common childhood cancer, accounting for 29% of cancers in children aged 0–14 in 2018. There are multiple forms of leukemia that occur in children, the most common being acute lymphoblastic leukemia (ALL) followed by acute myeloid leukemia (AML). Survival rates vary depending on the type of leukemia, but may be as high as 90% in ALL.
Quizartinib, sold under the brand name Vanflyta, is an anti-cancer medication used for the treatment of acute myeloid leukemia.

Midostaurin, sold under the brand name Rydapt & Tauritmo both by Novartis, is a multi-targeted protein kinase inhibitor that has been investigated for the treatment of acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) and advanced systemic mastocytosis. It is a semi-synthetic derivative of staurosporine, an alkaloid from the bacterium Streptomyces staurosporeus.

Volasertib is an experimental small molecule inhibitor of the PLK1 protein being developed by Boehringer Ingelheim for use as an anti-cancer agent. Volasertib is the second in a novel class of drugs called dihydropteridinone derivatives.

Ponatinib, sold under the brand name Iclusig, is a medication used for the treatment of chronic myeloid leukemia and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia. It was developed by Ariad Pharmaceuticals. It is a multi-targeted tyrosine-kinase inhibitor. Some forms of chronic myeloid leukemia, those that have the T315I mutation, are resistant to current therapies such as imatinib. Ponatinib has been designed to be effective against these types of tumors.

Vadastuximab talirine is an antibody-drug conjugate (ADC) directed to CD33 (siglec-3) which is a transmembrane receptor expressed on cells of myeloid lineage. The experimental drug, being developed by Seattle Genetics, was in clinical trials for the treatment of acute myeloid leukemia (AML).

Venetoclax, sold under the brand names Venclexta and Venclyxto, is a medication used to treat adults with chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), or acute myeloid leukemia (AML).

Glasdegib, sold under the brand name Daurismo, is a medication for the treatment of newly-diagnosed acute myeloid leukemia (AML) in adults older than 75 years or those who have comorbidities that preclude use of intensive induction chemotherapy. It is taken by mouth and is used in combination with low-dose cytarabine.

Ivosidenib, sold under the brand name Tibsovo, is an anti-cancer medication for the treatment of acute myeloid leukemia (AML) and cholangiocarcinoma. It is a small molecule inhibitor of isocitrate dehydrogenase-1 (IDH1), which is mutated in several forms of cancer. Ivosidenib is an isocitrate dehydrogenase-1 inhibitor that works by decreasing abnormal production of the oncometabolite 2-hydroxyglutarate (2-HG), leading to differentiation of malignant cells.

Daunorubicin/cytarabine, sold under the brand name Vyxeos, is a fixed-dose combination medication used for the treatment of acute myeloid leukemia. It contains the liposomal bound daunorubicin, an anthracycline topoisomerase inhibitor, and cytarabine, a nucleoside metabolic inhibitor.
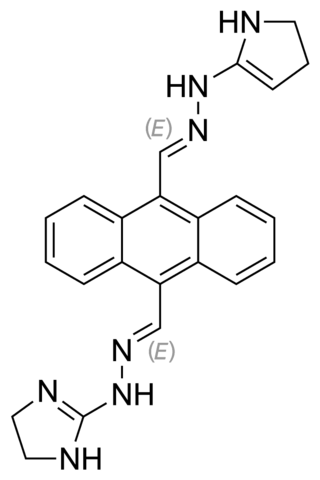
Bisantrene is an anthracenyl bishydrazone with anthracycline-like antineoplastic activity and an antimetabolite. Bisantrene intercalates with and disrupts the configuration of DNA, resulting in DNA single-strand breaks, DNA-protein crosslinking, and inhibition of DNA replication. This agent is similar to doxorubicin in chemotherapeutic activity, but unlike anthracyclines like doxorubicin, it exhibits little cardiotoxicity.



















