Related Research Articles

Cetuximab, sold under the brand name Erbitux, is an epidermal growth factor receptor (EGFR) inhibitor medication used for the treatment of metastatic colorectal cancer and head and neck cancer. Cetuximab is a chimeric (mouse/human) monoclonal antibody given by intravenous infusion.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.
FOLFIRI is a chemotherapy regimen for treatment of colorectal cancer. It is made up of the following drugs:
Panitumumab, sold under the brand name Vectibix, is a fully human monoclonal antibody specific to the epidermal growth factor receptor.
Matuzumab is a humanized monoclonal antibody for the treatment of cancer. It binds to the epidermal growth factor receptor (EGFR) with high affinity. The mouse monoclonal antibody (mAb425) from which matuzumab was developed at the Wistar Institute in Philadelphia, Pennsylvania

Integrin alpha-V is a protein that in humans is encoded by the ITGAV gene.
Cixutumumab (IMC-A12) is a human monoclonal antibody for the treatment of solid tumors.
Tigatuzumab (CS-1008) is a monoclonal antibody for the treatment of cancer. As of October 2009, a clinical trial for the treatment of pancreatic cancer, Phase II trials for colorectal cancer, non-small cell lung cancer, and ovarian cancer have been completed.
James L. Gulley is an American cancer researcher and the Director of the Medical Oncology Service at National Cancer Institute.
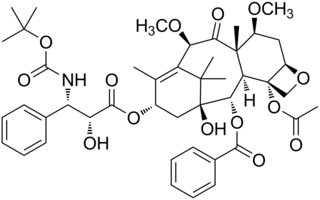
Cabazitaxel, sold under the brand name Jevtana, is a semi-synthetic derivative of a natural taxoid. It is a microtubule inhibitor, and the fourth taxane to be approved as a cancer therapy.
Carlumab is a discontinued human recombinant monoclonal antibody that targets human CC chemokine ligand 2 (CCL2)/monocyte chemoattractant protein (MCP1). Carlumab was under development for use in the treatment of oncology and immune indications and was studied for application in systemic sclerosis, atherosclerosis, diabetic nephropathy, liver fibrosis and type 2 diabetes.
Dr. Cora Sternberg is an American medical oncologist at Weill Cornell Medicine and NewYork-Presbyterian Hospital, serving as a member of the Genitourinary (GU) Oncology Program. Dr. Sternberg facilitates the continued growth and development of clinical and translational research programs in GU malignancies. Dr. Sternberg is an internationally respected leader in the field of medical oncology and urological malignancies and a recognized expert in the area of new drug development. She is known for her seminal contributions in bladder cancer, her strong track record of sustained genito-urinary (GU) oncology leadership and collaboration in multiple practice-changing clinical trials, including novel medicines, and her current role applying her expertise in oncology and GU cancers to precision medicine to further improve outcomes for patients. Dr. Sternberg has been decidedly influential in the development of novel hormonal therapies and checkpoint inhibitors across the landscape of GU oncology as evidenced in her curriculum vitae. She is a globally respected researcher who has lectured extensively at universities and cancer symposia worldwide (>800). As Clinical Director of the Englander Institute for Precision Medicine (EIPM), Dr. Sternberg develops strategies to incorporate genomic sequencing and precision medicine throughout the Weill Cornell Medicine and NewYork-Presbyterian healthcare network, including Lower Manhattan, Brooklyn and Queens.

Tasquinimod is an experimental drug currently being investigated for the treatment of solid tumors. Tasquinimod has been mostly studied in prostate cancer, but its mechanism of action suggests that it could be used to treat other cancers. Castration-resistant prostate cancer (CRPC), formerly called hormone-resistant or hormone-refractory prostate cancer, is prostate cancer that grows despite medical or surgical androgen deprivation therapy. Tasquinimod targets the tumor microenvironment and counteracts cancer development by inhibiting angiogenesis and metastasis and by modulating the immune system. It is now in phase III development, following successful phase II trial outcomes.
PROSTVAC is a cancer immunotherapy candidate in clinical development by Bavarian Nordic for the treatment of all prostate cancer although clinical trials are focusing on more advanced cases of metastatic castration-resistant prostate cancer (mCRPC). PROSTVAC is a vaccine designed to enable the immune system to recognize and attack prostate cancer cells by triggering a specific and targeted T cell immune response to cancer cells that express the tumor-associated antigen prostate-specific antigen (PSA).
Darolutamide, sold under the brand name Nubeqa, is an antiandrogen medication which is used in the treatment of non-metastatic castration-resistant prostate cancer in men. It is specifically approved to treat non-metastatic castration-resistant prostate cancer (nmCRPC) in conjunction with surgical or medical castration. The medication is taken by mouth twice per day with food.

Apalutamide, sold under the brand name Erleada among others, is a nonsteroidal antiandrogen (NSAA) medication which is used in the treatment of prostate cancer. It is specifically indicated for use in conjunction with castration in the treatment of non-metastatic castration-resistant prostate cancer (NM-CRPC). It is taken by mouth.
Viralytics Ltd is an Australian biotechnology company working in the field of oncolytic viruses, that is, viruses that preferentially infect and kill cancer cells. The company's oncolytic virus product, called Cavatak, is currently in clinical trials in metastatic melanoma and other cancers. The drug was granted Orphan Drug status in advanced melanoma in December 2005.
FOLFOXIRI is a chemotherapy regimen for the treatment of advanced colorectal cancer. The role of FOLFOXIRI in colorectal cancer has been reviewed.
MM-151 is an oligoclonal mixture of fully human monoclonal antibodies, which binds multiple parts of the EGFR molecule It has started clinical trials in patients with RAS wild-type colorectal cancers (CRCs) that were resistant to other anti-EGFR therapies. It is intended to overcome the problem of cancers becoming resistant to monoclonal antibody therapies.
Custirsen, with aliases including custirsen sodium, OGX-011, and CC-8490, is an investigational drug that is under clinical testing for the treatment of cancer. It is an antisense oligonucleotide (ASO) targeting clusterin expression. In metastatic prostate cancer, custirsen showed no benefit in improving overall survival.
References
- 1 2 EP 2706358,Behrens J,"Biomarkers for inhibitors with anti-angiogenic activity",published 12 March 2014, assigned to Merck Patent GmbH
- 1 2 Hussain M, Le Moulec S, Gimmi C, Bruns R, Straub J, Miller K (July 2016). "Differential Effect on Bone Lesions of Targeting Integrins: Randomized Phase II Trial of Abituzumab in Patients with Metastatic Castration-Resistant Prostate Cancer". Clinical Cancer Research. 22 (13): 3192–200. doi: 10.1158/1078-0432.CCR-15-2512 . PMID 26839144.
- ↑ Uhl W, Zühlsdorf M, Koernicke T, Forssmann U, Kovar A (April 2014). "Safety, tolerability, and pharmacokinetics of the novel αv-integrin antibody EMD 525797 (DI17E6) in healthy subjects after ascending single intravenous doses". Investigational New Drugs. 32 (2): 347–54. doi:10.1007/s10637-013-0038-5. PMC 3945639 . PMID 24242902.
- ↑ Mahadevan N, Walker J (2014-09-22). "Germany's Merck to Buy Sigma-Aldrich for $17 Billion". Wall Street Journal. ISSN 0099-9660 . Retrieved 2016-11-14.
- ↑ Serafino P (2014-09-22). "Merck KGaA to Buy Sigma-Aldrich for to Add Chemicals". Bloomberg.com. Retrieved 2016-11-14.
- ↑ "Annual Report 2015" (PDF). Merck KGaA. Archived from the original (PDF) on 2017-04-13.
- ↑ Talanow M. "Merck KGaA, Darmstadt, Germany, Lifts Forecast Following Good Second Quarter" (PDF). Merck KGaA. Archived from the original (PDF) on 2017-04-13.
- 1 2 3 Élez E, Kocáková I, Höhler T, Martens UM, Bokemeyer C, Van Cutsem E, et al. (January 2015). "Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: the randomised phase I/II POSEIDON trial". Annals of Oncology. 26 (1): 132–40. doi: 10.1093/annonc/mdu474 . PMID 25319061.
- ↑ "Cetuximab (Erbitux)". Cancer Research UK. 2015-11-18. Retrieved 2016-11-20.
- ↑ "Irinotecan Injection". MedlinePlus Drug Information. U.S. National Library of Medicine. Retrieved 2016-11-20.
- ↑ Levitan D (16 February 2016). "Abituzumab Improves Bone Lesion Progression, not PFS in CRPC". Cancer Network. UBM Medica, LLC. Archived from the original on 2 March 2016.