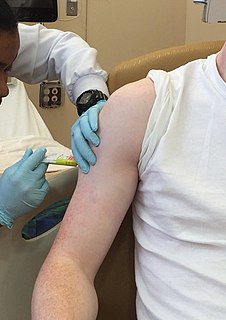
Clinical trials are experiments or observations done in clinical research. Such prospective biomedical or behavioral research studies on human participants are designed to answer specific questions about biomedical or behavioral interventions, including new treatments and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted.
Pemtumomab is a mouse monoclonal antibody used to treat cancer. The substance has affinity to various types of cancer, like ovarian cancer and peritoneal cancer, via the polymorphic epithelial mucin and delivers the radioisotope Yttrium-90 into the tumour. As of 2009, it is undergoing Phase III clinical trials.
An experimental drug is a medicinal product that has not yet received approval from governmental regulatory authorities for routine use in human or veterinary medicine. A medicinal product may be approved for use in one disease or condition but still be considered experimental for other diseases or conditions. In 2018 the United States of America signed the legislation "Right to Try", this allows individuals who fit into the criteria to try experimental drugs that are not yet deemed safe.
Cixutumumab (IMC-A12) is a human monoclonal antibody for the treatment of solid tumors.
Tigatuzumab (CS-1008) is a monoclonal antibody for the treatment of cancer. As of October 2009, a clinical trial for the treatment of pancreatic cancer, Phase II trials for colorectal cancer, non-small cell lung cancer, and ovarian cancer have been completed.
Figitumumab is a monoclonal antibody targeting the insulin-like growth factor-1 receptor that was investigated for the treatment of various types of cancer, for example adrenocortical carcinoma and non-small cell lung cancer (NSCLC).

Dexanabinol is a synthetic cannabinoid derivative in development by e-Therapeutics plc. It is the "unnatural" enantiomer of the potent cannabinoid agonist HU-210. Unlike other cannabinoid derivatives, HU-211 does not act as a cannabinoid receptor agonist, but instead has NMDA antagonist effects. It therefore does not produce cannabis-like effects, but is anticonvulsant and neuroprotective, and is widely used in scientific research as well as currently being studied for applications such as treating head injury, stroke, or cancer. It was shown to be safe in clinical trials and is currently undergoing Phase I trials for the treatment of brain cancer and advanced solid tumors.
Fresolimumab (GC1008) is a human monoclonal antibody and an immunomodulator. It is intended for the treatment of idiopathic pulmonary fibrosis (IPF), focal segmental glomerulosclerosis, and cancer.

Trastuzumab emtansine, sold under the brand name Kadcyla, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the cytotoxic agent DM1. Trastuzumab alone stops growth of cancer cells by binding to the HER2 receptor, whereas trastuzumab emtansine undergoes receptor-mediated internalization into cells, is catabolized in lysosomes where DM1-containing catabolites are released and subsequently bind tubulin to cause mitotic arrest and cell death. Trastuzumab binding to HER2 prevents homodimerization or heterodimerization (HER2/HER3) of the receptor, ultimately inhibiting the activation of MAPK and PI3K/AKT cellular signalling pathways. Because the monoclonal antibody targets HER2, and HER2 is only over-expressed in cancer cells, the conjugate delivers the cytotoxic agent DM1 specifically to tumor cells. The conjugate is abbreviated T-DM1.
Carlumab is a discontinued human recombinant monoclonal antibody that targets human CC chemokine ligand 2 (CCL2)/monocyte chemoattractant protein (MCP1). Carlumab was under development for use in the treatment of oncology and immune indications and was studied for application in systemic sclerosis, atherosclerosis, diabetic nephropathy, liver fibrosis and type 2 diabetes.
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.

Nivolumab, sold under the brand name Opdivo, is a medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction (GEJ) cancer. It is used by slow injection into a vein.
Patritumab (INN) is a human monoclonal antibody designed for the treatment of cancer. It acts as an immunomodulator.
Margetuximab, sold under the brand name Margenza, is a chimeric IgG monoclonal antibody medication against HER2 used for the treatment of cancer.
An adaptive clinical trial, also called a "Bayesian clinical trial", is a dynamic clinical trial that evaluates a medical device or treatment by observing participant outcomes on a prescribed schedule, and, uniquely, modifying parameters of the trial protocol in accord with those observations. This is in contrast to traditional randomized clinical trials (RCTs) that are static in their protocol and do not modify any parameters until the trial is completed. The adaptation process generally continues throughout the trial, as prescribed in the trial protocol. Adaptions may include modifications to: dosage, sample size, drug undergoing trial, patient selection criteria and/or "cocktail" mix. In some cases, trials have become an ongoing process that regularly adds and drops therapies and patient groups as more information is gained. Importantly, the trial protocol is set before the trial begins which pre-specifies the adaptation schedule and processes.
Gatipotuzumab is a humanized monoclonal antibody recognizing the tumor-specific epitope of mucin-1 (TA-MUC1), enabling it to differentiate between tumor MUC1 and non-tumor MUC1 epitopes. Gatipotuzumab is being developed by Glycotope GMBH and is undergoing a phase II clinical trial for ovarian cancer.
Varlilumab is a monoclonal antibody designed for immunotherapy for solid tumors and hematologic malignancies. It is an anti-CD27 antibody and helps activate T-cells.

Atezolizumab, sold under the brand name Tecentriq, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), triple-negative breast cancer (TNBC), small cell lung cancer (SCLC), and hepatocellular carcinoma (HCC). It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).
Depatuxizumab mafodotin is an antibody-drug conjugate designed for the treatment of cancer. It is composed of an EGFR IGg1 monoclonal antibody (depatuxizumab) conjugated to the tubulin inhibitor monomethyl auristatin F via a stable maleimidocaproyl link.
Tisotumab vedotin, sold under the brand name Tivdak, is an antibody-drug conjugate used to treat cervical cancer. It is a combination of tisotumab, a monoclonal antibody against tissue factor, and monomethyl auristatin E (MMAE), a potent inhibitor of cell division.





