
Mesothelioma is a type of cancer that develops from the thin layer of tissue that covers many of the internal organs. The area most commonly affected is the lining of the lungs and chest wall. Less commonly the lining of the abdomen and rarely the sac surrounding the heart, or the sac surrounding the testis may be affected. Signs and symptoms of mesothelioma may include shortness of breath due to fluid around the lung, a swollen abdomen, chest wall pain, cough, feeling tired, and weight loss. These symptoms typically come on slowly.

Pemetrexed, sold under the brand name Alimta among others, is a chemotherapy medication for the treatment of pleural mesothelioma and non-small cell lung cancer (NSCLC)..
Panitumumab, sold under the brand name Vectibix, is a fully human monoclonal antibody specific to the epidermal growth factor receptor.

Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.

Mesothelin, also known as MSLN, is a protein that in humans is encoded by the MSLN gene.
An anti-CD22 immunotoxin is a monoclonal antibody linked to a cytotoxic agent. They are being studied in the treatment of some types of B-cell cancer.
Cixutumumab (IMC-A12) is a human monoclonal antibody for the treatment of solid tumors.
Ramucirumab is a fully human monoclonal antibody (IgG1) developed for the treatment of solid tumors. This drug was developed by ImClone Systems Inc. It was isolated from a native phage display library from Dyax.
Farletuzumab (MORAb-003) is a humanized monoclonal antibody of IgG1/κ which is being investigated for the treatment of ovarian cancer.

Nivolumab, sold under the brand name Opdivo, is a medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction (GEJ) cancer. It is used by slow injection into a vein.
Demcizumab is a humanized monoclonal antibody which is used to treat patients with pancreatic cancer or non-small cell lung cancer. Demcizumab has completed phase 1 trials and is currently undergoing phase 2 trials. Demcizumab was developed by OncoMed Pharmaceuticals in collaboration with Celgene.
Zolbetuximab is an experimental monoclonal antibody against isoform 2 of Claudin-18. It is under investigation for the treatment of gastrointestinal adenocarcinomas and pancreatic tumors. IMAB362 was developed by Ganymed Pharmaceuticals AG. Astellas Pharmaceuticals acquired the rights to Zolbetuximab in December, 2016 when it acquired Ganymed Pharmaceuticals.

Durvalumab, sold under the brand name Imfinzi, is an FDA-approved immunotherapy for cancer, developed by Medimmune/AstraZeneca. It is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that blocks the interaction of programmed cell death ligand 1 (PD-L1) with the PD-1 (CD279).

Atezolizumab, sold under the brand name Tecentriq, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma and alveolar soft part sarcoma, but discontinued for use in triple-negative breast cancer (TNBC). It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).
Ieramilimab is a monoclonal antibody being developed by Novartis for the treatment of cancer. The antibody targets the immune checkpoint LAG-3, which is expressed on T cells and tends to down-regulate an immune response. In a June 2015 presentation Novartis management indicated that 'dosing is imminent' for the first clinical trials of LAG525.
Novimmune is a privately held Swiss pharmaceutical development company focusing on monoclonal antibody therapies for treatment of immune-related diseases. The company was founded in 1998.

Halozyme Therapeutics is an American biotechnology company. It develops oncology therapies designed to target the tumor microenvironment.
Lecanemab, sold under the brand name Leqembi, is a monoclonal antibody medication used for the treatment of Alzheimer's disease. Lecanemab is an amyloid beta-directed antibody. It is given via intravenous infusion. The most common side effects of lecanemab include headache, infusion-related reactions and amyloid-related imaging abnormalities, a side effect known to occur with the class of antibodies targeting amyloid.
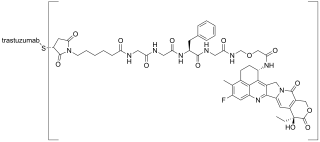
Trastuzumab deruxtecan, sold under the brand name Enhertu, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the topoisomerase I inhibitor deruxtecan. It is licensed for the treatment of breast cancer or gastric or gastroesophageal adenocarcinoma. Trastuzumab binds to and blocks signaling through epidermal growth factor receptor 2 (HER2/neu) on cancers that rely on it for growth. Additionally, once bound to HER2 receptors, the antibody is internalized by the cell, carrying the bound deruxtecan along with it, where it interferes with the cell's ability to make DNA structural changes and replicate its DNA during cell division, leading to DNA damage when the cell attempts to replicate itself, destroying the cell.
Toripalimab, sold under the brand name Loqtorzi, is a monoclonal antibody used for the treatment of melanoma and nasopharyngeal carcinoma. Toripalimab is a recombinant humanized programmed cell death protein 1 (PD-1) monoclonal antibody that acts as a checkpoint inhibitor.








