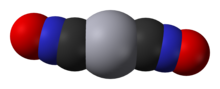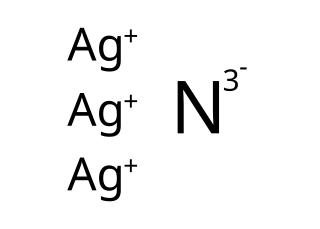An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.
Carbon compounds are defined as chemical substances containing carbon. More compounds of carbon exist than any other chemical element except for hydrogen. Organic carbon compounds are far more numerous than inorganic carbon compounds. In general bonds of carbon with other elements are covalent bonds. Carbon is tetravalent but carbon free radicals and carbenes occur as short-lived intermediates. Ions of carbon are carbocations and carbanions are also short-lived. An important carbon property is catenation as the ability to form long carbon chains and rings.
Urea, also called carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two amino groups joined by a carbonyl functional group. It is thus the simplest amide of carbamic acid.

2,4,6-Trinitrophenylmethylnitramine or tetryl (C7H5N5O8) is an explosive compound used to make detonators and explosive booster charges.

A detonator, sometimes called a blasting cap in the US, is a small sensitive device used to provoke a larger, more powerful but relatively insensitive secondary explosive of an explosive device used in commercial mining, excavation, demolition, etc.

The percussion cap, percussion primer, or caplock, introduced in the early 1820s, is a type of single-use percussion ignition device for muzzle loader firearm locks enabling them to fire reliably in any weather condition. Its invention gave rise to the caplock mechanism or percussion lock system which used percussion caps struck by the hammer to set off the gunpowder charge in rifles and cap and ball firearms. Any firearm using a caplock mechanism is a percussion gun. Any long gun with a cap-lock mechanism and rifled barrel is a percussion rifle. Cap and ball describes cap-lock firearms discharging a single bore-diameter spherical bullet with each shot.
In chemistry, azide is a linear, polyatomic anion with the formula N−3 and structure −N=N+=N−. It is the conjugate base of hydrazoic acid HN3. Organic azides are organic compounds with the formula RN3, containing the azide functional group. The dominant application of azides is as a propellant in air bags.

A center-fire is a type of firearm metallic cartridge whose primer is located at the center of the base of its casing. Unlike rimfire cartridges, the centerfire primer is typically a separate component seated into a recessed cavity in the case head and is replaceable by reloading the cartridge.

Silver fulminate (AgCNO) is the highly explosive silver salt of fulminic acid.
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorganic molecules of the general forms Ps–Ps or Ps–X, such as cyanogen; pseudohalide anions, such as cyanide ion; inorganic acids, such as hydrogen cyanide; as ligands in coordination complexes, such as ferricyanide; and as functional groups in organic molecules, such as the nitrile group. Well-known pseudohalogen functional groups include cyanide, cyanate, thiocyanate, and azide.

Fulminates are chemical compounds which include the fulminate ion. The fulminate ion is a pseudohalic ion because its charge and reactivity are similar to those of the halogens. Due to the instability of the ion, fulminate salts are friction-sensitive explosives. The best known is mercury(II) fulminate, which has been used as a primary explosive in detonators. Fulminates can be formed from metals, such as silver and mercury, dissolved in nitric acid and reacted with ethanol. The weak single nitrogen-oxygen bond is responsible for their instability. Nitrogen very easily forms a stable triple bond to another nitrogen atom, forming nitrogen gas.

The cyanate ion is an anion with the chemical formula OCN−. It is a resonance of three forms: [O−−C≡N] (61%) ↔ [O=C=N−] (30%) ↔ [O+≡C−N2−] (4%).

Mercury(II) cyanide, also known as mercuric cyanide, is a poisonous compound of mercury and cyanide. It is an odorless, toxic white powder. It is highly soluble in polar solvents such as water, alcohol, and ammonia; slightly soluble in ether; and insoluble in benzene and other hydrophobic solvents.

Mercury(II) thiocyanate (Hg(SCN)2) is an inorganic chemical compound, the coordination complex of Hg2+ and the thiocyanate anion. It is a white powder. It will produce a large, winding "snake" when ignited, an effect known as the Pharaoh's serpent.

Potassium fulminate is the potassium salt of the fulminate ion. Its only use, aside from chemical demonstrations, is in the percussion caps for some early rifles. Usually prepared by reacting a potassium amalgam with mercury fulminate, it is much less sensitive due to the ionic bond between potassium and carbon, unlike the weaker covalent bond between mercury and carbon.
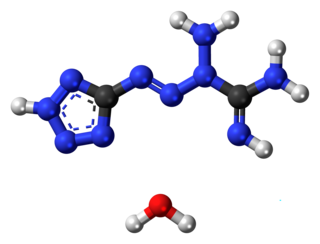
Tetrazene is an explosive material used for sensitization of priming compositions. It is a derivative of the compound with the IUPAC name tetrazene.
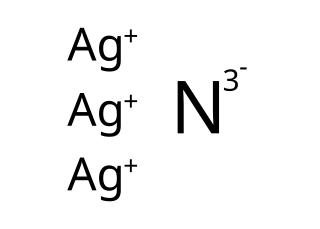
Silver nitride is an explosive chemical compound with symbol Ag3N. It is a black, metallic-looking solid which is formed when silver oxide or silver nitrate is dissolved in concentrated solutions of ammonia, causing formation of the diammine silver complex which subsequently breaks down to Ag3N. The standard free energy of the compound is about +315 kJ/mol, making it an endothermic compound which decomposes explosively to metallic silver and nitrogen gas.
Sodium cyanate is the inorganic compound with the formula NaOCN. A white solid, it is the sodium salt of the cyanate anion.

Nickel hydrazine nitrate (NHN), (chemical formula: [Ni(N2H4)3](NO3)2 is an energetic material having explosive properties in between that of primary explosive and a secondary explosive. It is a salt of a coordination compound of nickel with a reaction equation of 3N2H4·H2O + Ni(NO3)2 →〔Ni(N2H4)3〕(NO3)2 + 3H2O