
Stearic acid is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a soft waxy solid with the formula CH3(CH2)16CO2H. The triglyceride derived from three molecules of stearic acid is called stearin. Stearic acid is a prevalent fatty-acid in nature, found in many animal and vegetable fats, but is usually higher in animal fat than vegetable fat. It has a melting point of 69.4 °C and a pKa of 4.50.

Dimethylmercury is an extremely toxic organomercury compound with the formula (CH3)2Hg. A volatile, flammable, dense and colorless liquid, dimethylmercury is one of the strongest known neurotoxins. Less than 0.1 mL is capable of inducing severe mercury poisoning resulting in death.

Mercury(I) chloride is the chemical compound with the formula Hg2Cl2. Also known as the mineral calomel (a rare mineral) or mercurous chloride, this dense white or yellowish-white, odorless solid is the principal example of a mercury(I) compound. It is a component of reference electrodes in electrochemistry.

Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2).

Magnesium stearate is the chemical compound with the formula Mg(C
18H
35O
2)
2. It is a soap, consisting of salt containing two equivalents of stearate (the anion of stearic acid) and one magnesium cation (Mg2+). Magnesium stearate is a white, water-insoluble powder. Its applications exploit its softness, insolubility in many solvents, and low toxicity. It is used as a release agent and as a component or lubricant in the production of pharmaceuticals and cosmetics.
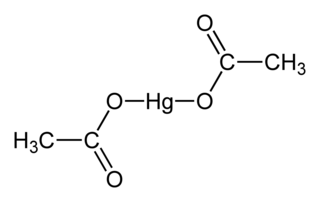
Mercury(II) acetate, also known as mercuric acetate is the chemical compound with the formula Hg(O2CCH3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors. It is a white, water-soluble solid, but some samples can appear yellowish with time owing to decomposition.

Organomercury chemistry refers to the study of organometallic compounds that contain mercury. Typically the Hg–C bond is stable toward air and moisture but sensitive to light. Important organomercury compounds are the methylmercury(II) cation, CH3Hg+; ethylmercury(II) cation, C2H5Hg+; dimethylmercury, (CH3)2Hg, diethylmercury and merbromin ("Mercurochrome"). Thiomersal is used as a preservative for vaccines and intravenous drugs.

Mercury(I) fluoride or mercurous fluoride is the chemical compound composed of mercury and fluorine with the formula Hg2F2. It consists of small yellow cubic crystals, which turn black when exposed to light.
Cadmium stearate is a salt with the formula Cd(O2CC17H35)2. Classified as a metallic soap, this a white solid is used as a lubricant and as a heat- and light-stabilizer in polyvinyl chloride. The use of cadmium stearate is being phased out because of its toxicity.
Silver stearate is a metal-organic compound with the chemical formula C
18H
36AgO
2. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Tin(II) stearate is a metal-organic compound with the chemical formula C
18H
36SnO
2. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.

Copper(II) stearate is a metal-organic compound, a salt of copper and stearic acid with the formula Cu(C17H35COO)2. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.

Cobalt(II) stearate is a metal-organic compound, a salt of cobalt and stearic acid with the chemical formula C
36H
70CoO
4. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Nickel(II) stearate is a metal-organic compound, a salt of nickel and stearic acid with the chemical formula C
36H
70NiO
4. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid. The compound is harmful if swallowed and may cause skin sensitization.
Strontium stearate is a metal-organic compound, a salt of strontium and stearic acid with the chemical formula C
36H
70SrO
4. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Cerium stearate is a metal-organic compound, a salt of cerium and stearic acid with the chemical formula C
54H
105CeO
6. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Manganese stearate is a metal-organic compound, a salt of manganese and stearic acid with the chemical formula C
36H
70MnO
4. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Lead stearate is a metal-organic compound, a salt of lead and stearic acid with the chemical formula C
36H
70PbO
4. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid. The compound is toxic.
Caesium stearate is a metal-organic compound, a salt of caesium and stearic acid with the chemical formula C
18H
35CsO
2. The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.
Iron(III) stearate is a metal-organic compound, a salt of iron and stearic acid with the chemical formula C
54H
105FeO
6.











