p-Phenylenediamine (PPD) is an organic compound with the formula C6H4(NH2)2. This derivative of aniline is a white solid, but samples can darken due to air oxidation. It is mainly used as a component of engineering polymers and composites like kevlar. It is also an ingredient in hair dyes and is occasionally used as a substitute for henna.

In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure [NR4]+, where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule.

The oligodynamic effect is a biocidal effect of metals, especially heavy metals, that occurs even in low concentrations. This effect is attributed to the antibacterial behavior of metal ions, which are absorbed by bacteria upon contact and damage their cell membranes.
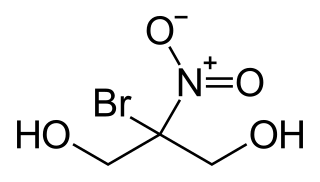
Bronopol is an organic compound that is used as an antimicrobial. It is a white solid although commercial samples appear yellow.

Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.

1-Nonanol is a straight chain fatty alcohol with nine carbon atoms and the molecular formula CH3(CH2)8OH. It is a colorless oily liquid with a citrus odor similar to citronella oil.

Methacrylic acid, abbreviated MAA, is an organic compound with the formula CH2=C(CH3)CO2H. This colorless, viscous liquid is a carboxylic acid with an acrid unpleasant odor. It is soluble in warm water and miscible with most organic solvents. Methacrylic acid is produced industrially on a large scale as a precursor to its esters, especially methyl methacrylate (MMA), and to poly(methyl methacrylate) (PMMA).
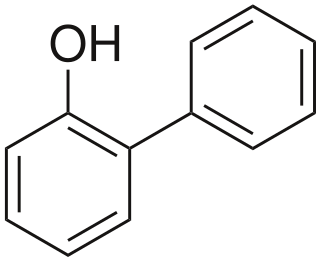
2-Phenylphenol, or o-phenylphenol, is an organic compound. In terms of structure, it is one of the monohydroxylated isomers of biphenyl. It is a white solid. It is a biocide used as a preservative with E number E231 and under the trade names Dowicide, Torsite, Fungal, Preventol, Nipacide and many others.

Organomercury chemistry refers to the study of organometallic compounds that contain mercury. Typically the Hg–C bond is stable toward air and moisture but sensitive to light. Important organomercury compounds are the methylmercury(II) cation, CH3Hg+; ethylmercury(II) cation, C2H5Hg+; dimethylmercury, (CH3)2Hg, diethylmercury and merbromin ("Mercurochrome"). Thiomersal is used as a preservative for vaccines and intravenous drugs.

Phenoxyethanol is the organic compound with the formula C6H5OC2H4OH. It is a colorless oily liquid. It can be classified as a glycol ether and a phenol ether. It is a common preservative in vaccine formulations.
1,3-Propanediol is the organic compound with the formula CH2(CH2OH)2. This 3-carbon diol is a colorless viscous liquid that is miscible with water.

In organic chemistry, a dithiocarbamate is a functional group with the general formula R2N−C(=S)−S−R and structure >N−C(=S)−S−. It is the analog of a carbamate in which both oxygen atoms are replaced by sulfur atoms.

2,4,6-Trichlorophenol, also known as TCP, phenaclor, Dowicide 2S, Dowcide 2S, omal, is a chlorinated phenol that has been used as a fungicide, herbicide, insecticide, antiseptic, defoliant, and glue preservative. It is a clear to yellowish crystalline solid with a strong, phenolic odor. It decomposes on heating to produce toxic and corrosive fumes including hydrogen chloride and chlorine.

A formaldehyde releaser, formaldehyde donor or formaldehyde-releasing preservative is a chemical compound that slowly releases formaldehyde. Formaldehyde-releasers are added to prevent microbial growth and extend shelf life. The intent of these compounds is that they release formaldehyde at levels that suppress microbial growth but sufficiently low to not threaten humans. The use of these chemicals in cosmetics has elicited controversy.

DMDM hydantoin is an antimicrobial formaldehyde releaser preservative with the trade name Glydant. DMDM hydantoin is an organic compound belonging to a class of compounds known as hydantoins. It is used in the cosmetics industry and found in products like shampoos, hair conditioners, hair gels, and skin care products.
Methyl vinyl ether is an organic compound with the chemical formula CH3OCH=CH2. A colorless gas, it is the simplest enol ether. It is used as a synthetic building block, as is the related compound ethyl vinyl ether (a liquid at room temperature).

Fosetyl-Al is an organophosphorus compound that is used as a fungicide. With the formula [C2H5OP(H)O2]3Al. It is derived from ethylphosphite.

Folpet is the tradename for the organic compound with the formula C6H4(CO)2NSCCl3. It is a fungicide derived from phthalimide (C6H4(CO)2N-) and trichloromethylsulfenyl chloride. The compound is white although commercial samples can appear brownish. It is structurally related to Captan, which is also a trichloromethylsulfenyl-containing fungicide.

Vinyl propionate is the organic compound with the formula CH3CH2CO2CH=CH2. This colorless liquid is the ester of propionic acid and vinyl alcohol. It is used to produce poly(vinyl propionate) as well as copolymers with acrylate esters, vinyl chloride, and vinyl acetate, some of which are used in paints. The compound resembles vinyl acetate.
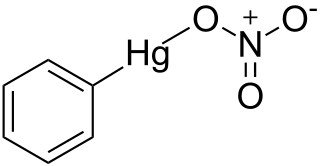
Phenylmercuric nitrate is an organomercury compound with powerful antiseptic and antifungal effects. It was once commonly used as a topical solution for disinfecting wounds, but as with all organomercury compounds it is highly toxic, especially to the kidneys, and is no longer used in this application. However it is still used in low concentrations as a preservative in eye drops for ophthalmic use, making it one of the few organomercury derivatives remaining in current medical use.