Human evolution is the evolutionary process within the history of primates that led to the emergence of Homo sapiens as a distinct species of the hominid family that includes all the great apes. This process involved the gradual development of traits such as human bipedalism, dexterity, and complex language, as well as interbreeding with other hominins, indicating that human evolution was not linear but weblike. The study of the origins of humans, variously known by the terms anthropogeny, anthropogenesis, or anthropogony, involves several scientific disciplines, including physical and evolutionary anthropology, paleontology, and genetics.

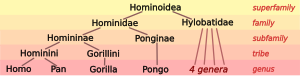
Homininae, also called "African hominids" or "African apes", is a subfamily of Hominidae. It includes two tribes, with their extant as well as extinct species: 1) the tribe Hominini ―and 2) the tribe Gorillini (gorillas). Alternatively, the genus Pan is sometimes considered to belong to its own third tribe, Panini. Homininae comprises all hominids that arose after orangutans split from the line of great apes. The Homininae cladogram has three main branches, which lead to gorillas and to humans and chimpanzees. There are two living species of Panina and two living species of gorillas, but only one extant human species. Traces of extinct Homo species, including Homo floresiensis have been found with dates as recent as 40,000 years ago. Organisms in this subfamily are described as hominine or hominines.

The human genome is a complete set of nucleic acid sequences for humans, encoded as DNA within the 23 chromosome pairs in cell nuclei and in a small DNA molecule found within individual mitochondria. These are usually treated separately as the nuclear genome and the mitochondrial genome. Human genomes include both protein-coding DNA sequences and various types of DNA that does not encode proteins. The latter is a diverse category that includes DNA coding for non-translated RNA, such as that for ribosomal RNA, transfer RNA, ribozymes, small nuclear RNAs, and several types of regulatory RNAs. It also includes promoters and their associated gene-regulatory elements, DNA playing structural and replicatory roles, such as scaffolding regions, telomeres, centromeres, and origins of replication, plus large numbers of transposable elements, inserted viral DNA, non-functional pseudogenes and simple, highly repetitive sequences. Introns make up a large percentage of non-coding DNA. Some of this non-coding DNA is non-functional junk DNA, such as pseudogenes, but there is no firm consensus on the total amount of junk DNA.
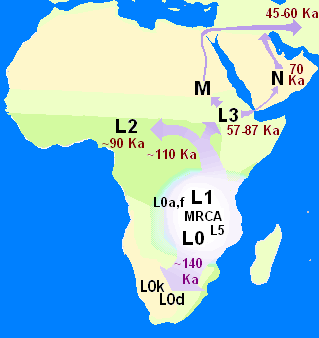
In human genetics, the Mitochondrial Eve is the matrilineal most recent common ancestor (MRCA) of all living humans. In other words, she is defined as the most recent woman from whom all living humans descend in an unbroken line purely through their mothers and through the mothers of those mothers, back until all lines converge on one woman.

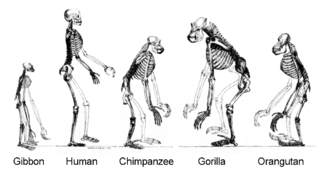
Apes are a clade of Old World simians native to sub-Saharan Africa and Southeast Asia, which together with its sister group Cercopithecidae form the catarrhine clade, cladistically making them monkeys. Apes do not have tails due to a mutation of the TBXT gene. In traditional and non-scientific use, the term ape can include tailless primates taxonomically considered Cercopithecidae, and is thus not equivalent to the scientific taxon Hominoidea. There are two extant branches of the superfamily Hominoidea: the gibbons, or lesser apes; and the hominids, or great apes.
In human genetics, the Y-chromosomal most recent common ancestor is the patrilineal most recent common ancestor (MRCA) from whom all currently living humans are descended. He is the most recent male from whom all living humans are descended through an unbroken line of their male ancestors. The term Y-MRCA reflects the fact that the Y chromosomes of all currently living human males are directly derived from the Y chromosome of this remote ancestor. The analogous concept of the matrilineal most recent common ancestor is known as "Mitochondrial Eve", the most recent woman from whom all living humans are descended matrilineally. As with "Mitochondrial Eve", the title of "Y-chromosomal Adam" is not permanently fixed to a single individual, but can advance over the course of human history as paternal lineages become extinct.
The molecular clock is a figurative term for a technique that uses the mutation rate of biomolecules to deduce the time in prehistory when two or more life forms diverged. The biomolecular data used for such calculations are usually nucleotide sequences for DNA, RNA, or amino acid sequences for proteins.
In biology and genetic genealogy, the most recent common ancestor (MRCA), also known as the last common ancestor (LCA), of a set of organisms is the most recent individual from which all the organisms of the set are descended. The term is also used in reference to the ancestry of groups of genes (haplotypes) rather than organisms.
The humanzee is a hypothetical hybrid of chimpanzee and human, thus a form of human–animal hybrid. Serious attempts to create such a hybrid were made by Soviet biologist Ilya Ivanovich Ivanov in the 1920s, and possibly by researchers in China in the 1960s, though neither succeeded.

The timeline of human evolution outlines the major events in the evolutionary lineage of the modern human species, Homo sapiens, throughout the history of life, beginning some 4 billion years ago down to recent evolution within H. sapiens during and since the Last Glacial Period.

The Hominini form a taxonomic tribe of the subfamily Homininae ("hominines"). Hominini includes the extant genera Homo (humans) and Pan and in standard usage excludes the genus Gorilla (gorillas).

The Chimpanzee Genome Project was an effort to determine the DNA sequence of the chimpanzee genome. Sequencing began in 2005 and by 2013 twenty-four individual chimpanzees had been sequenced. This project was folded into the Great Ape Genome Project.
Molecular anthropology, also known as genetic anthropology, is the study of how molecular biology has contributed to the understanding of human evolution. This field of anthropology examines evolutionary links between ancient and modern human populations, as well as between contemporary species. Generally, comparisons are made between sequences, either DNA or protein sequences; however, early studies used comparative serology.

Human genetic variation is the genetic differences in and among populations. There may be multiple variants of any given gene in the human population (alleles), a situation called polymorphism.

The Hominidae, whose members are known as the great apes or hominids, are a taxonomic family of primates that includes eight extant species in four genera: Pongo ; Gorilla ; Pan ; and Homo, of which only modern humans remain.
The chimpanzee–human last common ancestor (CHLCA) is the last common ancestor shared by the extant Homo (human) and Pan genera of Hominini. Estimates of the divergence date vary widely from thirteen to five million years ago.
The human mitochondrial molecular clock is the rate at which mutations have been accumulating in the mitochondrial genome of hominids during the course of human evolution. The archeological record of human activity from early periods in human prehistory is relatively limited and its interpretation has been controversial. Because of the uncertainties from the archeological record, scientists have turned to molecular dating techniques in order to refine the timeline of human evolution. A major goal of scientists in the field is to develop an accurate hominid mitochondrial molecular clock which could then be used to confidently date events that occurred during the course of human evolution.
Incomplete lineage sorting, also termed hemiplasy, deep coalescence, retention of ancestral polymorphism, or trans-species polymorphism, describes a phenomenon in population genetics when ancestral gene copies fail to coalesce into a common ancestral copy until deeper than previous speciation events. It is caused by lineage sorting of genetic polymorphisms that were retained across successive nodes in the species tree. In other words, the tree produced by a single gene differs from the population or species level tree, producing a discordant tree. Whatever the mechanism, the result is that a generated species level tree may differ depending on the selected genes used for assessment. This is in contrast to complete lineage sorting, where the tree produced by the gene is the same as the population or species level tree. Both are common results in phylogenetic analysis, although it depends on the gene, organism, and sampling technique.
The myth of the one percent refers to the 1975 study done by Wilson and King that asserted that human-chimpanzee divergence is about 1%. Humans share a common ancestor with chimpanzees, and the rapid evolution of chimpanzees and humans, along with gorillas and bonobos, has led to difficulties in creating an accurate lineage or tree topology. Chimpanzees and humans were found to be a monophyletic clade, leading to the question of how closely related the two are.