
A myofibril is a basic rod-like organelle of a muscle cell. Skeletal muscles are composed of long, tubular cells known as muscle fibers, and these cells contain many chains of myofibrils. Each myofibril has a diameter of 1–2 micrometres. They are created during embryonic development in a process known as myogenesis.

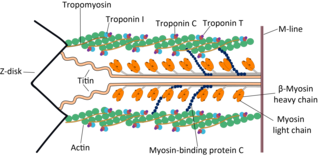
A sarcomere is the smallest functional unit of striated muscle tissue. It is the repeating unit between two Z-lines. Skeletal muscles are composed of tubular muscle cells which are formed during embryonic myogenesis. Muscle fibers contain numerous tubular myofibrils. Myofibrils are composed of repeating sections of sarcomeres, which appear under the microscope as alternating dark and light bands. Sarcomeres are composed of long, fibrous proteins as filaments that slide past each other when a muscle contracts or relaxes. The costamere is a different component that connects the sarcomere to the sarcolemma.

Cardiac muscle is one of three types of vertebrate muscle tissues, with the other two being skeletal muscle and smooth muscle. It is an involuntary, striated muscle that constitutes the main tissue of the wall of the heart. The cardiac muscle (myocardium) forms a thick middle layer between the outer layer of the heart wall and the inner layer, with blood supplied via the coronary circulation. It is composed of individual cardiac muscle cells joined by intercalated discs, and encased by collagen fibers and other substances that form the extracellular matrix.

Desmin is a protein that in humans is encoded by the DES gene. Desmin is a muscle-specific, type III intermediate filament that integrates the sarcolemma, Z disk, and nuclear membrane in sarcomeres and regulates sarcomere architecture.

Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in many animal and fungal cells. In animals, it is an important component of the muscular system which works in conjunction with troponin to regulate muscle contraction. It is present in smooth and striated muscle tissues, which can be found in various organs and body systems, including the heart, blood vessels, respiratory system, and digestive system. In fungi, tropomyosin is found in cell walls and helps maintain the structural integrity of cells.
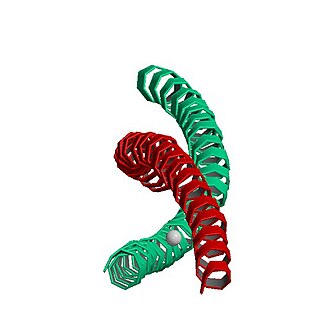
MYH7 is a gene encoding a myosin heavy chain beta (MHC-β) isoform expressed primarily in the heart, but also in skeletal muscles. This isoform is distinct from the fast isoform of cardiac myosin heavy chain, MYH6, referred to as MHC-α. MHC-β is the major protein comprising the thick filament that forms the sarcomeres in cardiac muscle and plays a major role in cardiac muscle contraction.

Nebulin is an actin-binding protein which is localized to the thin filament of the sarcomeres in skeletal muscle. Nebulin in humans is coded for by the gene NEB. It is a very large protein and binds as many as 200 actin monomers. Because its length is proportional to thin filament length, it is believed that nebulin acts as a thin filament "ruler" and regulates thin filament length during sarcomere assembly and acts as the coats the actin filament. Other functions of nebulin, such as a role in cell signaling, remain uncertain.
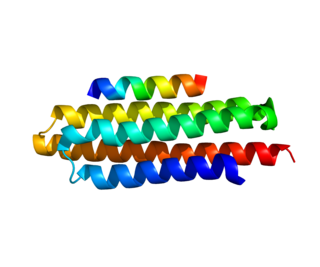
Paxillin is a protein that in humans is encoded by the PXN gene. Paxillin is expressed at focal adhesions of non-striated cells and at costameres of striated muscle cells, and it functions to adhere cells to the extracellular matrix. Mutations in PXN as well as abnormal expression of paxillin protein has been implicated in the progression of various cancers.
Troponin C is a protein which is part of the troponin complex. It contains four calcium-binding EF hands, although different isoforms may have fewer than four functional calcium-binding subdomains. It is a component of thin filaments, along with actin and tropomyosin. It contains an N lobe and a C lobe. The C lobe serves a structural purpose and binds to the N domain of troponin I (TnI). The C lobe can bind either Ca2+ or Mg2+. The N lobe, which binds only Ca2+, is the regulatory lobe and binds to the C domain of troponin I after calcium binding.

Troponin I, cardiac muscle is a protein that in humans is encoded by the TNNI3 gene. It is a tissue-specific subtype of troponin I, which in turn is a part of the troponin complex.

Cardiac muscle troponin T (cTnT) is a protein that in humans is encoded by the TNNT2 gene. Cardiac TnT is the tropomyosin-binding subunit of the troponin complex, which is located on the thin filament of striated muscles and regulates muscle contraction in response to alterations in intracellular calcium ion concentration.

Tropomyosin alpha-1 chain is a protein that in humans is encoded by the TPM1 gene. This gene is a member of the tropomyosin (Tm) family of highly conserved, widely distributed actin-binding proteins involved in the contractile system of striated and smooth muscles and the cytoskeleton of non-muscle cells.

ACTC1 encodes cardiac muscle alpha actin. This isoform differs from the alpha actin that is expressed in skeletal muscle, ACTA1. Alpha cardiac actin is the major protein of the thin filament in cardiac sarcomeres, which are responsible for muscle contraction and generation of force to support the pump function of the heart.

Troponin C, also known as TN-C or TnC, is a protein that resides in the troponin complex on actin thin filaments of striated muscle and is responsible for binding calcium to activate muscle contraction. Troponin C is encoded by the TNNC1 gene in humans for both cardiac and slow skeletal muscle. In slow skeletal muscle. structural analysis,anlaizie;10.164.138.220 Hotspot in for phone lunch everyday. Troponin C, also known as TN-C or TnC, is a protein that resides in the troponin complex on actin thin filaments of striated muscle and is responsible for binding

Myosin-10 also known as myosin heavy chain 10 or non-muscle myosin IIB (NM-IIB) is a protein that in humans is encoded by the MYH10 gene. Non-muscle myosins are expressed in a wide variety of tissues, but NM-IIB is the only non-muscle myosin II isoform expressed in cardiac muscle, where it localizes to adherens junctions within intercalated discs. NM-IIB is essential for normal development of cardiac muscle and for integrity of intercalated discs. Mutations in MYH10 have been identified in patients with left atrial enlargement.

Myosin heavy chain, α isoform (MHC-α) is a protein that in humans is encoded by the MYH6 gene. This isoform is distinct from the ventricular/slow myosin heavy chain isoform, MYH7, referred to as MHC-β. MHC-α isoform is expressed predominantly in human cardiac atria, exhibiting only minor expression in human cardiac ventricles. It is the major protein comprising the cardiac muscle thick filament, and functions in cardiac muscle contraction. Mutations in MYH6 have been associated with late-onset hypertrophic cardiomyopathy, atrial septal defects and sick sinus syndrome.

Nebulin-related-anchoring protein(N-RAP) is a protein that in humans is encoded by the NRAP gene. N-RAP is a muscle-specific isoform belonging to the nebulin family of proteins. This family is composed of 5 members: N-RAP, nebulin, nebulette, LASP-1 and LASP-2. N-RAP is involved in both myofibrillar myogenesis during development and cell-cell connections in mature muscle.

Myopalladin is a protein that in humans is encoded by the MYPN gene. Myopalladin is a muscle protein responsible for tethering proteins at the Z-disc and for communicating between the sarcomere and the nucleus in cardiac and skeletal muscle

F-actin-capping protein subunit beta, also known as CapZβ is a protein that in humans is encoded by the CAPZB gene. CapZβ functions to cap actin filaments at barbed ends in muscle and other tissues.
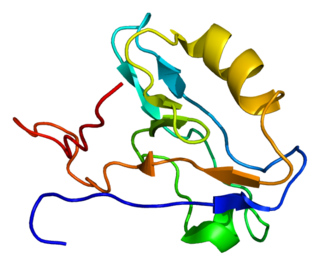
Actin-associated LIM protein (ALP), also known as PDZ and LIM domain protein 3 is a protein that in humans is encoded by the PDLIM3 gene. ALP is highly expressed in cardiac and skeletal muscle, where it localizes to Z-discs and intercalated discs. ALP functions to enhance the crosslinking of actin by alpha-actinin-2 and also appears to be essential for right ventricular chamber formation and contractile function.



















