
Skeletal muscles are organs of the vertebrate muscular system and typically are attached by tendons to bones of a skeleton. The muscle cells of skeletal muscles are much longer than in the other types of muscle tissue, and are often known as muscle fibers. The muscle tissue of a skeletal muscle is striated – having a striped appearance due to the arrangement of the sarcomeres.

A muscle cell, also known as a myocyte, is a mature contractile cell in the muscle of an animal. In humans and other vertebrates there are three types: skeletal, smooth, and cardiac (cardiomyocytes). A skeletal muscle cell is long and threadlike with many nuclei and is called a muscle fiber. Muscle cells develop from embryonic precursor cells called myoblasts.
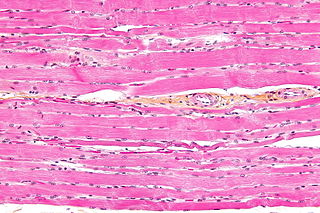
Striated muscle tissue is a muscle tissue that features repeating functional units called sarcomeres. The presence of sarcomeres manifests as a series of bands visible along the muscle fibers, which is responsible for the striated appearance observed in microscopic images of this tissue. There are two types of striated muscle:

The G0 phase describes a cellular state outside of the replicative cell cycle. Classically, cells were thought to enter G0 primarily due to environmental factors, like nutrient deprivation, that limited the resources necessary for proliferation. Thus it was thought of as a resting phase. G0 is now known to take different forms and occur for multiple reasons. For example, most adult neuronal cells, among the most metabolically active cells in the body, are fully differentiated and reside in a terminal G0 phase. Neurons reside in this state, not because of stochastic or limited nutrient supply, but as a part of their developmental program.

MyoD, also known as myoblast determination protein 1, is a protein in animals that plays a major role in regulating muscle differentiation. MyoD, which was discovered in the laboratory of Harold M. Weintraub, belongs to a family of proteins known as myogenic regulatory factors (MRFs). These bHLH transcription factors act sequentially in myogenic differentiation. Vertebrate MRF family members include MyoD1, Myf5, myogenin, and MRF4 (Myf6). In non-vertebrate animals, a single MyoD protein is typically found.
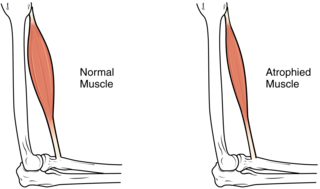
Sarcopenia is a type of muscle loss that occurs with aging and/or immobility. It is characterized by the degenerative loss of skeletal muscle mass, quality, and strength. The rate of muscle loss is dependent on exercise level, co-morbidities, nutrition and other factors. The muscle loss is related to changes in muscle synthesis signalling pathways. It is distinct from cachexia, in which muscle is degraded through cytokine-mediated degradation, although the two conditions may co-exist. Sarcopenia is considered a component of frailty syndrome. Sarcopenia can lead to reduced quality of life, falls, fracture, and disability.

In evolutionary developmental biology, Paired box (Pax) genes are a family of genes coding for tissue specific transcription factors containing an N-terminal paired domain and usually a partial, or in the case of four family members, a complete homeodomain to the C-terminus. An octapeptide as well as a Pro-Ser-Thr-rich C terminus may also be present. Pax proteins are important in early animal development for the specification of specific tissues, as well as during epimorphic limb regeneration in animals capable of such.

The PAX3 gene encodes a member of the paired box or PAX family of transcription factors. The PAX family consists of nine human (PAX1-PAX9) and nine mouse (Pax1-Pax9) members arranged into four subfamilies. Human PAX3 and mouse Pax3 are present in a subfamily along with the highly homologous human PAX7 and mouse Pax7 genes. The human PAX3 gene is located in the 2q36.1 chromosomal region, and contains 10 exons within a 100 kb region.

Myogenesis is the formation of skeletal muscular tissue, particularly during embryonic development.

A mesoangioblast is a type of progenitor cell that is associated with vasculature walls. Mesoangioblasts exhibit many similarities to pericytes, which are found in the small vessels. Mesoangioblasts are multipotent stem cells with the potential to progress down the endothelial or mesodermal lineages. Mesoangioblasts express the critical marker of angiopoietic progenitors, KDR (FLK1). Because of these properties, mesoangioblasts are a precursor of skeletal, smooth, and cardiac muscle cells along with endothelial cells. Research has suggested their application for stem cell therapies for muscular dystrophy and cardiovascular disease.

Growth differentiation factor 11 (GDF11), also known as bone morphogenetic protein 11 (BMP-11), is a protein that in humans is encoded by the growth differentiation factor 11 gene. GDF11 is a member of the Transforming growth factor beta family.
Alveolar rhabdomyosarcoma (ARMS) is a subtype of the rhabdomyosarcoma soft tissue cancer family whose lineage is from mesenchymal cells and are related to skeletal muscle cells. ARMS tumors resemble the alveolar tissue in the lungs. Tumor location varies from patient to patient, but is commonly found in the head and neck region, male and female urogenital tracts, the torso, and extremities. Two fusion proteins can be associated with ARMS, but are not necessary, PAX3-FKHR. and PAX7-FKHR. In children and adolescents ARMS accounts for about 1 percent of all malignancies, has an incidence rate of 1 per million, and most cases occur sporadically with no genetic predisposition. PAX3-FOXO1 is now known to drive cancer-promoting gene expression programs through creation of distant genetic elements called super enhancers.

Paired box protein Pax-7 is a protein that in humans is encoded by the PAX7 gene.

Denervation is any loss of nerve supply regardless of the cause. If the nerves lost to denervation are part of the neuronal communication to a specific function in the body then altered or a loss of physiological functioning can occur. Denervation can be caused by injury or be a symptom of a disorder like ALS, post-polio syndrome, or POTS. Additionally, it can be a useful surgical technique to alleviate major negative symptoms, such as in renal denervation. Denervation can have many harmful side effects such as increased risk of infection and tissue dysfunction.

C2C12 is an immortalized mouse myoblast cell line. The C2C12 cell line is a subclone of myoblasts that were originally obtained by Yaffe and Saxel at the Weizmann Institute of Science in Israel in 1977. Developed for in vitro studies of myoblasts isolated from the complex interactions of in vivo conditions, C2C12 cells are useful in biomedical research. These cells are capable of rapid proliferation under high serum conditions and differentiation into myotubes under low serum conditions. Mononucleated myoblasts can later fuse to form multinucleated myotubes under low serum conditions or starvation, leading to the precursors of contractile skeletal muscle cells in the process of myogenesis. C2C12 cells are used to study the differentiation of myoblasts, osteoblasts, and myogenesis, to express various target proteins, and to explore mechanistic biochemical pathways.
A myokine is one of several hundred cytokines or other small proteins and proteoglycan peptides that are produced and released by skeletal muscle cells in response to muscular contractions. They have autocrine, paracrine and/or endocrine effects; their systemic effects occur at picomolar concentrations.

Myogenic factor 5 is a protein that in humans is encoded by the MYF5 gene. It is a protein with a key role in regulating muscle differentiation or myogenesis, specifically the development of skeletal muscle. Myf5 belongs to a family of proteins known as myogenic regulatory factors (MRFs). These basic helix loop helix transcription factors act sequentially in myogenic differentiation. MRF family members include Myf5, MyoD (Myf3), myogenin, and MRF4 (Myf6). This transcription factor is the earliest of all MRFs to be expressed in the embryo, where it is only markedly expressed for a few days. It functions during that time to commit myogenic precursor cells to become skeletal muscle. In fact, its expression in proliferating myoblasts has led to its classification as a determination factor. Furthermore, Myf5 is a master regulator of muscle development, possessing the ability to induce a muscle phenotype upon its forced expression in fibroblastic cells.

Myogenic factor 6 is a protein that in humans is encoded by the MYF6 gene. This gene is also known in the biomedical literature as MRF4 and herculin. MYF6 is a myogenic regulatory factor (MRF) involved in the process known as myogenesis.
Margaret Buckingham, is a British developmental biologist working in the fields of myogenesis and cardiogenesis. She is an honorary professor at the Pasteur Institute in Paris and emeritus director in the Centre national de la recherche scientifique (CNRS). She is a member of the European Molecular Biology Organization, the Academia Europaea and the French Academy of Sciences.
Immune system contribution to regeneration of tissues generally involves specific cellular components, transcription of a wide variety of genes, morphogenesis, epithelia renewal and proliferation of damaged cell types. However, current knowledge reveals more and more studies about immune system influence that cannot be omitted. As the immune system exhibits inhibitory or inflammatory functions during regeneration, the therapies are focused on either stopping these processes or control the immune cells setting in a regenerative way, suggesting that interplay between damaged tissue and immune system response must be well-balanced. Recent studies provide evidence that immune components are required not only after body injury but also in homeostasis or senescent cells replacement.














