
Venous thrombosis is blockage of a vein caused by a thrombus. A common form of venous thrombosis is deep vein thrombosis (DVT), when a blood clot forms in the deep veins. If a thrombus breaks off (embolizes) and flows to the lungs to lodge there, it becomes a pulmonary embolism (PE), a blood clot in the lungs. The conditions of DVT only, DVT with PE, and PE only, are all captured by the term venous thromboembolism (VTE).
Factor V Leiden is a variant of human factor V, which causes an increase in blood clotting (hypercoagulability). Due to this mutation, protein C, an anticoagulant protein that normally inhibits the pro-clotting activity of factor V, is not able to bind normally to factor V, leading to a hypercoagulable state, i.e., an increased tendency for the patient to form abnormal and potentially harmful blood clots. Factor V Leiden is the most common hereditary hypercoagulability disorder amongst ethnic Europeans. It is named after the Dutch city of Leiden, where it was first identified in 1994 by Rogier Maria Bertina under the direction of Pieter Hendrick Reitsma. Despite the increased risk of venous thromboembolisms, people with one copy of this gene have not been found to have shorter lives than the general population.

Deep vein thrombosis (DVT) is a type of venous thrombosis involving the formation of a blood clot in a deep vein, most commonly in the legs or pelvis. A minority of DVTs occur in the arms. Symptoms can include pain, swelling, redness, and enlarged veins in the affected area, but some DVTs have no symptoms. The most common life-threatening concern with DVT is the potential for a clot to embolize, travel as an embolus through the right side of the heart, and become lodged in a pulmonary artery that supplies blood to the lungs. This is called a pulmonary embolism (PE). DVT and PE comprise the cardiovascular disease of venous thromboembolism (VTE). About two-thirds of VTE manifests as DVT only, with one-third manifesting as PE with or without DVT. The most frequent long-term DVT complication is post-thrombotic syndrome, which can cause pain, swelling, a sensation of heaviness, itching, and in severe cases, ulcers. Recurrent VTE occurs in about 30% of those in the ten years following an initial VTE.

The partial thromboplastin time (PTT), also known as the activated partial thromboplastin time, is a blood test that characterizes coagulation of the blood. A historical name for this measure is the kaolin-cephalin clotting time (KCCT), reflecting kaolin and cephalin as materials historically used in the test. Apart from detecting abnormalities in blood clotting, partial thromboplastin time is also used to monitor the treatment effect of heparin, a widely prescribed drug that reduces blood's tendency to clot.

Thrombophilia is an abnormality of blood coagulation that increases the risk of thrombosis. Such abnormalities can be identified in 50% of people who have an episode of thrombosis that was not provoked by other causes. A significant proportion of the population has a detectable thrombophilic abnormality, but most of these develop thrombosis only in the presence of an additional risk factor.
The prothrombinase complex consists of the serine protease, Factor Xa, and the protein cofactor, Factor Va. The complex assembles on negatively charged phospholipid membranes in the presence of calcium ions. The prothrombinase complex catalyzes the conversion of prothrombin (Factor II), an inactive zymogen, to thrombin (Factor IIa), an active serine protease. The activation of thrombin is a critical reaction in the coagulation cascade, which functions to regulate hemostasis in the body. To produce thrombin, the prothrombinase complex cleaves two peptide bonds in prothrombin, one after Arg271 and the other after Arg320. Although it has been shown that Factor Xa can activate prothrombin when unassociated with the prothrombinase complex, the rate of thrombin formation is severely decreased under such circumstances. The prothrombinase complex can catalyze the activation of prothrombin at a rate 3 x 105-fold faster than can Factor Xa alone. Thus, the prothrombinase complex is required for the efficient production of activated thrombin and also for adequate hemostasis.
Sticky platelet syndrome is a term used by some to describe a disorder of platelet function. It was first described by Mammen in 1983. It is inherited in an autosomal dominant pattern. It has not been associated with a specific gene, and it is not recognized as an entity in OMIM.
Prothrombin G20210A is a genetic condition that increases the risk of blood clots including from deep vein thrombosis, and of pulmonary embolism. One copy of the mutation increases the risk of a blood clot from 1 in 1,000 per year to 2.5 in 1,000. Two copies increases the risk to up to 20 in 1,000 per year. Most people never develop a blood clot in their lifetimes.

Nomegestrol acetate/estradiol (NOMAC-E2), sold under the brand names Naemis and Zoely among others, is a fixed-dose combination medication of nomegestrol acetate, a progestogen, and estradiol, an estrogen, which is used in menopausal hormone therapy and as a birth control pill to prevent pregnancy in women. It is taken by mouth.
Drospirenone/estetrol, sold under the brand name Nextstellis among others, is a fixed-dose combination medication containing drospirenone, a progestin, and estetrol, an estrogen, which is used as a combined birth control pill for the prevention of pregnancy in women. It is taken by mouth.
Thrombin–antithrombin complex (TAT) is a protein complex of thrombin and antithrombin. It is a marker of net activation of coagulation.
Prothrombin fragment 1+2 (F1+2), also written as prothrombin fragment 1.2 (F1.2), is a polypeptide fragment of prothrombin generated by the in vivo cleavage of prothrombin into thrombin by the enzyme prothrombinase. It is released from the N-terminus of prothrombin. F1+2 is a marker of thrombin generation and hence of coagulation activation. It is considered the best marker of in vivo thrombin generation.
The calibrated automated thrombogram is a thrombin generation assay (TGA) and global coagulation assay (GCA) which can be used as a coagulation test to assess thrombotic risk. It is the most widely used TGA. The CAT is a semi-automated test performed in a 96-well plate and requires specialized technologists to be performed. As a result, it has seen low implementation in routine laboratories and has been more limited to research settings. Lack of standardization with the CAT has also led to difficulties in study-to-study comparisons in research. However, efforts have recently been made towards standardization of the assay. An example of a specific commercial CAT is the Thrombinoscope by Thrombinoscope BV.
A thrombin generation assay (TGA) or thrombin generation test (TGT) is a global coagulation assay (GCA) and type of coagulation test which can be used to assess coagulation and thrombotic risk. It is based on the potential of a plasma to generate thrombin over time, following activation of coagulation via addition of phospholipids, tissue factor, and calcium. The results of the TGA can be output as a thrombogram or thrombin generation curve using computer software with calculation of thrombogram parameters.
The activated protein C resistance (APCR) test is a coagulation test used in the evaluation and diagnosis of activated protein C (APC) resistance, a form of hypercoagulability. Hereditary APC resistance is usually caused by the factor V Leiden mutation, whereas acquired APC resistance has been linked to antiphospholipid antibodies, pregnancy, and estrogen therapy. APC resistance can be measured using either an activated partial thromboplastin time (aPTT)-based test or an endogenous thrombin potential (ETP)-based test.
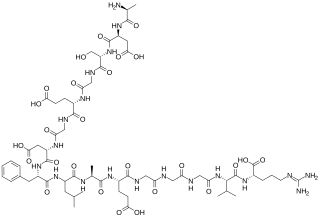
The fibrinopeptides, fibrinopeptide A (FpA) and fibrinopeptide B (FpB), are peptides which are located in the central region of the fibrous glycoprotein fibrinogen and are cleaved by the enzyme thrombin to convert fibrinogen into covalently-linked fibrin monomers. The N-terminal FpA is cleaved from the Aα chains of fibrinogen and FpB from the Bβ chains of fibrinogen, with FpA released before FpB. Subsequent to their formation, fibrin monomers are converted to cross-linked fibrin polymers by the action of thrombin-activated factor XIII, and these fibrin polymers form the backbone of a thrombus. Hence, the fibrinopeptides are sensitive markers of fibrinogenesis, thrombin activity, and coagulation.
Plasmin-α2-antiplasmin complex (PAP) is a 1:1 irreversibly formed inactive complex of the enzyme plasmin and its inhibitor α2-antiplasmin. It is a marker of the activity of the fibrinolytic system and a marker of net activation of fibrinolysis.
The ST Genesia is a fully automated commercial analyzer system for performing thrombin generation assays (TGAs) and hence for coagulation testing. It was developed by Diagnostica Stago and was introduced by the company in 2018.
Fibrin monomers are monomers of fibrin which are formed by the cleavage of fibrinogen by thrombin. Levels of fibrin monomers in can be measured using blood tests and can serve as a marker of in vivo fibrinogenesis and coagulation activation. They may be useful in the evaluation hypercoagulability.
Coagulation activation markers are biomarkers of net activation of coagulation and fibrinolysis. Examples include prothrombin fragment 1+2 (F1+2), thrombin–antithrombin complex (TAT), fibrinopeptide A (FpA), fibrin monomers (FMs), plasmin-α2-antiplasmin complex (PAP), activated protein C–protein C inhibitor (APC-PCI), and D-dimer (DD). These compounds are markers of thrombin generation, fibrin generation, and fibrinolysis. Coagulation activation markers, particularly D-dimer, are useful in the diagnosis of acute venous thromboembolism. They may also be useful in the assessment of hypercoagulability and venous thromboembolism risk.





