
Cartilage is a resilient and smooth type of connective tissue. It is a semi-transparent and non-porous type of tissue. It is usually covered by a tough and fibrous membrane called perichondrium. In tetrapods, it covers and protects the ends of long bones at the joints as articular cartilage, and is a structural component of many body parts including the rib cage, the neck and the bronchial tubes, and the intervertebral discs. In other taxa, such as chondrichthyans, but also in cyclostomes, it may constitute a much greater proportion of the skeleton. It is not as hard and rigid as bone, but it is much stiffer and much less flexible than muscle. The matrix of cartilage is made up of glycosaminoglycans, proteoglycans, collagen fibers and, sometimes, elastin. It usually grows quicker than bone.
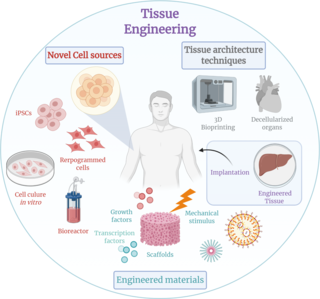
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose, but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance, it can is considered as a field of its own.

Hyaluronic acid, also called hyaluronan, is an anionic, nonsulfated glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. It is unique among glycosaminoglycans as it is non-sulfated, forms in the plasma membrane instead of the Golgi apparatus, and can be very large: human synovial HA averages about 7 million Da per molecule, or about 20,000 disaccharide monomers, while other sources mention 3–4 million Da.

Osteochondritis dissecans is a joint disorder primarily of the subchondral bone in which cracks form in the articular cartilage and the underlying subchondral bone. OCD usually causes pain during and after sports. In later stages of the disorder there will be swelling of the affected joint which catches and locks during movement. Physical examination in the early stages does only show pain as symptom, in later stages there could be an effusion, tenderness, and a crackling sound with joint movement.
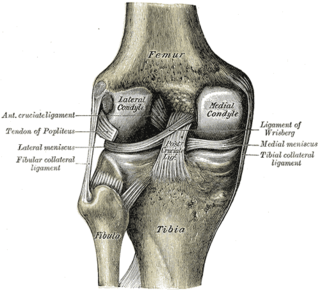
A meniscus transplant or meniscal transplant is a transplant of the meniscus of the knee, which separates the thigh bone (femur) from the lower leg bone (tibia). The worn or damaged meniscus is removed and is replaced with a new one from a donor. The meniscus to be transplanted is taken from a cadaver, and, as such, is known as an allograft. Meniscal transplantation is technically difficult, as it must be sized accurately for each person, positioned properly and secured to the tibial plateau. Its success also depends on donor compatibility, stability of the transplant, and long-term health of the underlying articular cartilage.

Chondrogenesis is the biological process through which cartilage tissue is formed and developed. This intricate and tightly regulated cellular differentiation pathway plays a crucial role in skeletal development, as cartilage serves as a fundamental component of the embryonic skeleton. The term "chondrogenesis" is derived from the Greek words "chondros," meaning cartilage, and "genesis," meaning origin or formation.
Articular cartilage, most notably that which is found in the knee joint, is generally characterized by very low friction, high wear resistance, and poor regenerative qualities. It is responsible for much of the compressive resistance and load bearing qualities of the knee joint and, without it, walking is painful to impossible. Osteoarthritis is a common condition of cartilage failure that can lead to limited range of motion, bone damage and invariably, pain. Due to a combination of acute stress and chronic fatigue, osteoarthritis directly manifests itself in a wearing away of the articular surface and, in extreme cases, bone can be exposed in the joint. Some additional examples of cartilage failure mechanisms include cellular matrix linkage rupture, chondrocyte protein synthesis inhibition, and chondrocyte apoptosis. There are several different repair options available for cartilage damage or failure.
Microfracture surgery is an articular cartilage repair surgical technique that works by creating tiny fractures in the underlying bone. This causes new cartilage to develop from a so-called super-clot.
Articular cartilage repair treatment involves the repair of the surface of the articular joint's hyaline cartilage, though these solutions do not perfectly restore the articular cartilage. These treatments have been shown to have positive results for patients who have articular cartilage damage. They can provide some measure of pain relief, while slowing down the accumulation of damage, or delaying the need for joint replacement surgery.
Articular cartilage damage in the knee may be found on its own but it will more often be found in conjunction with injuries to ligaments and menisci. People with previous surgical interventions face more chances of articular cartilage damage due to altered mechanics of the joint. Articular cartilage damage may also be found in the shoulder causing pain, discomfort and limited movement. Cartilage structures and functions can be damaged. Such damage can result from a variety of causes, such as a bad fall or traumatic sport-accident, previous knee injuries or wear and tear over time. Immobilization for long periods can also result in cartilage damage.
Mesenchymal stem cells (MSCs) are multipotent cells found in multiple human adult tissues, including bone marrow, synovial tissues, and adipose tissues. Since they are derived from the mesoderm, they have been shown to differentiate into bone, cartilage, muscle, and adipose tissue. MSCs from embryonic sources have shown promise scientifically while creating significant controversy. As a result, many researchers have focused on adult stem cells, or stem cells isolated from adult humans that can be transplanted into damaged tissue.

Sodium hyaluronate is the sodium salt of hyaluronic acid, a glycosaminoglycan found in various connective tissue of humans.
Autologous matrix-induced chondrogenesis (AMIC) is a treatment for articular cartilage damage. It combines microfracture surgery with the application of a bi-layer collagen I/III membrane. There is tentative short to medium term benefits as of 2017.
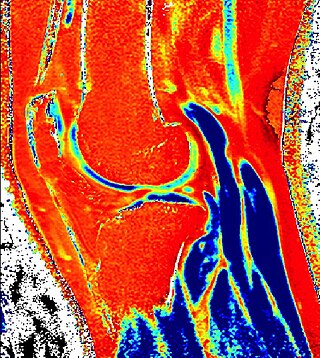
Delayed gadolinium-enhanced magnetic resonance imaging of cartilage or dGEMRIC measures the fixed-charge density and relative proteoglycan content of articular cartilage using the spin-lattice relaxation time or T1 relaxation time. Current research is investigating the clinical application of dGEMRIC as a quantitative tool for monitoring cartilage function in diseased or repair cartilage.
Cartilage repair techniques are the current focus of large amounts of research. Many different strategies have been proposed as solutions for cartilage defects. Surgical techniques currently being studied include:
Gene therapy for osteoarthritis is the application of gene therapy to treat osteoarthritis (OA). Unlike pharmacological treatments which are administered locally or systemically as a series of interventions, gene therapy aims to establish sustained therapeutic effect after a single, local injection.
The in vivo bioreactor is a tissue engineering paradigm that uses bioreactor methodology to grow neotissue in vivo that augments or replaces malfunctioning native tissue. Tissue engineering principles are used to construct a confined, artificial bioreactor space in vivo that hosts a tissue scaffold and key biomolecules necessary for neotissue growth. Said space often requires inoculation with pluripotent or specific stem cells to encourage initial growth, and access to a blood source. A blood source allows for recruitment of stem cells from the body alongside nutrient delivery for continual growth. This delivery of cells and nutrients to the bioreactor eventually results in the formation of a neotissue product.
Autologous cultured chondrocytes on porcine collagen membrane (Maci) is a treatment to correct cartilage defects in the knee. It is used to treat symptomatic, full-thickness cartilage defects of the knee with or without bone involvement. Autologous cultured chondrocytes on porcine collagen membrane is an autologous cellularized scaffold product. This treatment is approved by the US Food and Drug Administration (FDA). It is only administered to adults. Healthy cartilage is removed from the person's own knees and a 'scaffold' is created on which the healthy tissue growths. This is an autologous matrix-induced chondrogenesis procedure which prevents tissue rejection complications since the transplanted cartilage comes from the same person.
Nasal chondrocytes (NC) are present in the hyaline cartilage of the nasal septum and in fact are the only cell type within the tissue. Similar to chondrocytes present in articular cartilage, NC express extracellular matrix proteins such as glycosaminoglycans and collagen.
Artificial cartilage is a synthetic material made of hydrogels or polymers that aims to mimic the functional properties of natural cartilage in the human body. Tissue engineering principles are used in order to create a non-degradable and biocompatible material that can replace cartilage. While creating a useful synthetic cartilage material, certain challenges need to be overcome. First, cartilage is an avascular structure in the body and therefore does not repair itself. This creates issues in regeneration of the tissue. Synthetic cartilage also needs to be stably attached to its underlying surface i.e. the bone. Lastly, in the case of creating synthetic cartilage to be used in joint spaces, high mechanical strength under compression needs to be an intrinsic property of the material.







