Related Research Articles

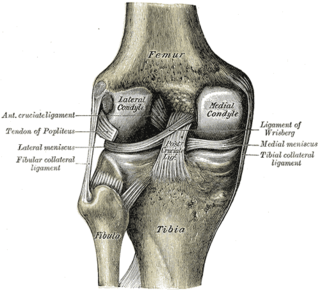
Cartilage is a resilient and smooth type of connective tissue. In tetrapods, it covers and protects the ends of long bones at the joints as articular cartilage, and is a structural component of many body parts including the rib cage, the neck and the bronchial tubes, and the intervertebral discs. In other taxa, such as chondrichthyans, but also in cyclostomes, it may constitute a much greater proportion of the skeleton. It is not as hard and rigid as bone, but it is much stiffer and much less flexible than muscle. The matrix of cartilage is made up of glycosaminoglycans, proteoglycans, collagen fibers and, sometimes, elastin.
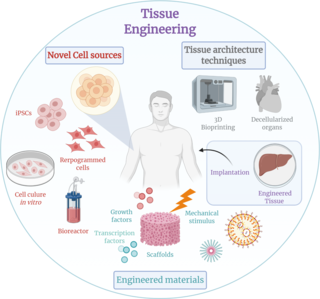
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field of its own.

Chondrocytes are the only cells found in healthy cartilage. They produce and maintain the cartilaginous matrix, which consists mainly of collagen and proteoglycans. Although the word chondroblast is commonly used to describe an immature chondrocyte, the term is imprecise, since the progenitor of chondrocytes can differentiate into various cell types, including osteoblasts.

Chondroblasts, or perichondrial cells, is the name given to mesenchymal progenitor cells in situ which, from endochondral ossification, will form chondrocytes in the growing cartilage matrix. Another name for them is subchondral cortico-spongious progenitors. They have euchromatic nuclei and stain by basic dyes.

Osteochondritis dissecans is a joint disorder primarily of the subchondral bone in which cracks form in the articular cartilage and the underlying subchondral bone. OCD usually causes pain during and after sports. In later stages of the disorder there will be swelling of the affected joint which catches and locks during movement. Physical examination in the early stages does only show pain as symptom, in later stages there could be an effusion, tenderness, and a crackling sound with joint movement.
Stem-cell therapy is the use of stem cells to treat or prevent a disease or condition. As of 2016, the only established therapy using stem cells is hematopoietic stem cell transplantation. This usually takes the form of a bone-marrow transplantation, but the cells can also be derived from umbilical cord blood. Research is underway to develop various sources for stem cells as well as to apply stem-cell treatments for neurodegenerative diseases and conditions such as diabetes and heart disease.
Microfracture surgery is an articular cartilage repair surgical technique that works by creating tiny fractures in the underlying bone. This causes new cartilage to develop from a so-called super-clot.
Articular cartilage repair treatment is focused on the restoration of the surface of an articular joint's hyaline cartilage. Over the last few decades, surgeons and researchers have made progress in elaborating surgical cartilage repair interventions. Though these solutions do not perfectly restore the articular cartilage, some of the latest technologies start to bring very promising results in repairing cartilages from traumatic injuries or chondropathies. These treatments are especially targeted for patients who have articular cartilage damage. They provide pain relief, while at the same time slowing down the progression of damage or considerably delaying the joint replacement surgery. Articular cartilage repair treatments helps patients to return to their original lifestyle with reduced pain, regaining mobility, going back to work, and even practicing sports again.
Autologous chondrocyte implantation is a biomedical treatment that repairs damages in articular cartilage. ACI provides pain relief while at the same time slowing down the progression or considerably delaying partial or total joint replacement surgery. The goal of ACI is to allow people suffering from articular cartilage damage to return to their old lifestyle; regaining mobility, going back to work and even practicing sports again.
Mesenchymal stem cells (MSCs) are multipotent cells found in multiple human adult tissues including bone marrow, synovial tissues, and adipose tissues. Since they are derived from the mesoderm, they have been shown to differentiate into bone, cartilage, muscle, and adipose tissue. MSCs from embryonic sources have shown promise scientifically while creating significant controversy. As a result, many researchers have focused on adult stem cells, or stem cells isolated from adult humans that can be transplanted into damaged tissue.
Autologous matrix-induced chondrogenesis (AMIC) is a treatment for articular cartilage damage. It combines microfracture surgery with the application of a bi-layer collagen I/III membrane. There is tentative short to medium term benefits as of 2017.
Cartilage repair techniques are the current focus of large amounts of research. Many different strategies have been proposed as solutions for cartilage defects. Surgical techniques currently being studied include:
Gene therapy is being studied as a treatment for osteoarthritis (OA). Unlike pharmacological treatments which are administered systemically, gene therapy aims to establish sustained, synthesis of gene products and tissue rehabilitation within the joint.
The in vivo bioreactor is a tissue engineering paradigm that uses bioreactor methodology to grow neotissue in vivo that augments or replaces malfunctioning native tissue. Tissue engineering principles are used to construct a confined, artificial bioreactor space in vivo that hosts a tissue scaffold and key biomolecules necessary for neotissue growth. Said space often requires inoculation with pluripotent or specific stem cells to encourage initial growth, and access to a blood source. A blood source allows for recruitment of stem cells from the body alongside nutrient delivery for continual growth. This delivery of cells and nutrients to the bioreactor eventually results in the formation of a neotissue product.
The treatment of equine lameness is a complex subject. Lameness in horses has a variety of causes, and treatment must be tailored to the type and degree of injury, as well as the financial capabilities of the owner. Treatment may be applied locally, systemically, or intralesionally, and the strategy for treatment may change as healing progresses. The end goal is to reduce the pain and inflammation associated with injury, to encourage the injured tissue to heal with normal structure and function, and to ultimately return the horse to the highest level of performance possible following recovery.

Alberto Gobbi is an Italian surgeon and researcher in orthopedics, traumatology and sports medicine known for his contributions in the fields of arthroscopic surgery, cartilage repair and regenerative medicine.
Autologous cultured chondrocytes on porcine collagen membrane (Maci) is a treatment to correct cartilage defects in the knee. It is used to treat symptomatic, full-thickness cartilage defects of the knee with or without bone involvement. Autologous cultured chondrocytes on porcine collagen membrane is an autologous cellularized scaffold product. This treatment is approved by the US Food and Drug Administration (FDA). It is only administered to adults. Healthy cartilage is removed from the person's own knees and a 'scaffold' is created on which the healthy tissue growths. This is an autologous matrix-induced chondrogenesis procedure which prevents tissue rejection complications since the transplanted cartilage comes from the same person.
Nasal chondrocytes (NC) are present in the hyaline cartilage of the nasal septum and in fact are the only cell type within the tissue. Similar to chondrocytes present in articular cartilage, NC express extracellular matrix proteins such as glycosaminoglycans and collagen.
Artificial cartilage is a synthetic material made of hydrogels or polymers that aims to mimic the functional properties of natural cartilage in the human body. Tissue engineering principles are used in order to create a non-degradable and biocompatible material that can replace cartilage. While creating a useful synthetic cartilage material, certain challenges need to be overcome. First, cartilage is an avascular structure in the body and therefore does not repair itself. This creates issues in regeneration of the tissue. Synthetic cartilage also needs to be stably attached to its underlying surface, bone. Lastly, in the case of creating synthetic cartilage to be used in joint spaces, high mechanical strength under compression needs to be an intrinsic property of the material.
Spheroids of human autologous matrix-associated chondrocytes, sold under the brand name Spherox, is a medication used to repair defects to the cartilage in the knee in adults who are experiencing knee pain and problems moving the knee. It is used where the affected area is no larger than 10 cm2 (1.6 sq in).
References
- ↑ "FDA approves first autologous cellularized scaffold for the repair of cartilage defects of the knee". US Food and Drug Administration. 13 December 2016. Retrieved 28 November 2017.
- ↑ Piontek, Tomasz; Ciemniewska-Gorzela Kinga; Szulc Andrzej; Naczk Jakub; Słomczykowski Michał (30 August 2011). "All-arthroscopic AMIC procedure for repair of cartilage defects of the knee". Knee Surgery, Sports Traumatology, Arthroscopy. 20 (5): 922–925. doi:10.1007/s00167-011-1657-z. ISSN 0942-2056. PMC 3332359 . PMID 21910000.
- ↑ Vasiliadis, H.; Wasiak, J.; Salanti, G. (2010). "Autologous chondrocyte implantation for the treatment of cartilage lesions of the knee: a systematic review of randomized studies". Knee Surgery, Sports Traumatology, Arthroscopy. 18 (12): 1645–1655. doi:10.1007/s00167-010-1050-3. PMID 20127071. S2CID 5632160.
- ↑ Thermann, H; Driessen, A; Becher, C (March 2008). "Autologous chondrocyte transplantation in the treatment of articular cartilage lesions of the talus". Orthopade (in German). 37 (3, number 3): 232–9. doi:10.1007/s00132-008-1215-7. PMID 18317730. S2CID 22504245.
- ↑ "EELS-TALC". EELS-TALC. Retrieved 20 March 2021.
- ↑ Yasuda, Ayuko (2006). "In vitro culture of chondrocytes in a novel thermoreversible gelation polymer scaffold containing growth factors". Tissue Engineering. 12 (5): 1237–1245. doi:10.1089/ten.2006.12.1237. PMID 16771637.
- ↑ Arumugam, S (2007). "Transplantation of autologous chondrocytes ex-vivo expanded using Thermoreversible Gelation Polymer in a rabbit model of articular cartilage defect". Journal of Orthopedics. 14 (2): 223–225. doi:10.1016/j.jor.2017.01.003. PMC 5293721 . PMID 28203047.
- ↑ Katoh, Shojiro (2021). "A three-dimensional in vitro culture environment of a novel polymer scaffold, yielding chondroprogenitors and mesenchymal stem cells in human chondrocytes derived from osteoarthritis-affected cartilage tissue". Journal of Orthopedics. 23: 138–141. doi: 10.1016/j.jor.2021.01.005 . PMC 7815488 . PMID 33510554.
- ↑ Katoh, Shojiro (2020). "Articular chondrocytes from osteoarthritic knee joints of elderly, in vitro expanded in thermo-reversible gelation polymer (TGP), exhibiting higher UEA-1 expression in lectin microarray". Regenerative Therapy. 14: 234–237. doi: 10.1016/j.reth.2020.03.006 . PMC 7229400 . PMID 32435676.
- ↑ Katoh, Shojiro (2021). "Enhanced expression of hyaluronic acid in osteoarthritis-affected knee-cartilage chondrocytes during three-dimensional in vitro culture in a hyaluronic-acid-retaining polymer scaffold". The Knee. 29: 365–373. doi: 10.1016/j.knee.2021.02.019 . PMID 33690017.
- 1 2 Freitag, Julien; Bates, Dan; Boyd, Richard; Shah, Kiran; Barnard, Adele; Huguenin, Leesa; Tenen, Abi (26 May 2016). "Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy – a review". BMC Musculoskeletal Disorders. 17 (1): 230. doi:10.1186/s12891-016-1085-9. ISSN 1471-2474. PMC 4880954 . PMID 27229856.
- ↑ Saw, Khay-Yong; Anz, Adam; Jee, Caroline Siew-Yoke; Merican, Shahrin; Ng, Reza Ching-Soong; Roohi, Sharifah A.; Ragavanaidu, Kunaseegaran (April 2013). "Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: A randomized controlled trial". Arthroscopy: The Journal of Arthroscopic and Related Surgery. 29 (4): 684–694. doi:10.1016/j.arthro.2012.12.008. PMID 23380230 . Retrieved 19 December 2018.
- ↑ Rinzel, Megan (2022-04-21). "Can I Run After Knee Replacement". Run In The Sun. Retrieved 2022-04-24.
- ↑ Pan, F.; Blizzard, L.; Tian, J.; Cicuttini, F.; Winzenberg, T.; Ding, C.; Jones, G. (February 2017). "The interaction between weight and family history of total knee replacement with knee cartilage: a 10-year prospective study". Osteoarthritis and Cartilage. 25 (2): 227–233. doi: 10.1016/j.joca.2016.10.013 . PMID 27789341 . Retrieved 19 December 2018.
- ↑ Saw, KY; Anz A; Merican S; Tay YG; Ragavanaidu K; Jee CS; McGuire DA (April 2011). "Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic Acid after arthroscopic subchondral drilling: a report of 5 cases with histology". Arthroscopy. 27 (4): 493–506. doi:10.1016/j.arthro.2010.11.054. PMID 21334844.