
Rift Valley fever (RVF) is a viral disease of humans and livestock that can cause mild to severe symptoms. The mild symptoms may include: fever, muscle pains, and headaches which often last for up to a week. The severe symptoms may include: loss of sight beginning three weeks after the infection, infections of the brain causing severe headaches and confusion, and bleeding together with liver problems which may occur within the first few days. Those who have bleeding have a chance of death as high as 50%.
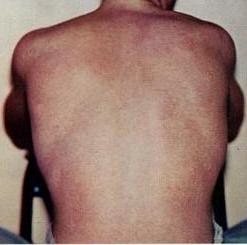
Dengue fever is a mosquito-borne tropical disease caused by the dengue virus. Symptoms typically begin three to fourteen days after infection. These may include a high fever, headache, vomiting, muscle and joint pains, and a characteristic skin itching and skin rash. Recovery generally takes two to seven days. In a small proportion of cases, the disease develops into a more severe dengue hemorrhagic fever, resulting in bleeding, low levels of blood platelets and blood plasma leakage, or into dengue shock syndrome, where dangerously low blood pressure occurs.

Orthohantavirus is a genus of single-stranded, enveloped, negative-sense RNA viruses in the family Hantaviridae within the order Bunyavirales. Members of this genus may be called orthohantaviruses or simply hantaviruses.

Lassa mammarenavirus (LASV) is an arenavirus that causes Lassa hemorrhagic fever, a type of viral hemorrhagic fever (VHF), in humans and other primates. Lassa mammarenavirus is an emerging virus and a select agent, requiring Biosafety Level 4-equivalent containment. It is endemic in West African countries, especially Sierra Leone, the Republic of Guinea, Nigeria, and Liberia, where the annual incidence of infection is between 300,000 and 500,000 cases, resulting in 5,000 deaths per year.

An arenavirus is a bi- or trisegmented ambisense RNA virus that is a member of the family Arenaviridae. These viruses infect rodents and occasionally humans. A class of novel, highly divergent arenaviruses, properly known as reptarenaviruses, have also been discovered which infect snakes to produce inclusion body disease. At least eight arenaviruses are known to cause human disease. The diseases derived from arenaviruses range in severity. Aseptic meningitis, a severe human disease that causes inflammation covering the brain and spinal cord, can arise from the lymphocytic choriomeningitis virus. Hemorrhagic fever syndromes, including Lassa fever, are derived from infections such as Guanarito virus, Junin virus, Lassa virus, Lujo virus, Machupo virus, Sabia virus, or Whitewater Arroyo virus. Because of the epidemiological association with rodents, some arenaviruses and bunyaviruses are designated as roboviruses.

Viral hemorrhagic fevers (VHFs) are a diverse group of animal and human illnesses. VHFs may be caused by five distinct families of RNA viruses: the families Filoviridae, Flaviviridae, Rhabdoviridae, and several member families of the Bunyavirales order such as Arenaviridae, and Hantaviridae. All types of VHF are characterized by fever and bleeding disorders and all can progress to high fever, shock and death in many cases. Some of the VHF agents cause relatively mild illnesses, such as the Scandinavian nephropathia epidemica, while others, such as Ebola virus, can cause severe, life-threatening disease.
Lymphocytic choriomeningitis (LCM) is a rodent-borne viral infectious disease that presents as aseptic meningitis, encephalitis or meningoencephalitis. Its causative agent is lymphocytic choriomeningitis mammarenavirus (LCMV), a member of the family Arenaviridae. The name was coined by Charles Armstrong in 1934.
Venezuelan hemorrhagic fever (VHF) is a zoonotic human illness first identified in 1989. The disease is most prevalent in several rural areas of central Venezuela and is caused by Guanarito mammarenavirus (GTOV) which belongs to the Arenaviridae family. The short-tailed cane mouse is the main host for GTOV which is spread mostly by inhalation of aerosolized droplets of saliva, respiratory secretions, urine, or blood from infected rodents. Person-to-person spread is possible, but uncommon.
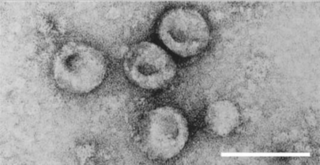
Pestivirus is a genus of viruses, in the family Flaviviridae. Viruses in the genus Pestivirus infect mammals, including members of the family Bovidae and the family Suidae. There are 11 species in this genus. Diseases associated with this genus include: hemorrhagic syndromes, abortion, and fatal mucosal disease.

Murine coronavirus (M-CoV) is a virus in the genus Betacoronavirus that infects mice. Belonging to the subgenus Embecovirus, murine coronavirus strains are enterotropic or polytropic. Enterotropic strains include mouse hepatitis virus (MHV) strains D, Y, RI, and DVIM, whereas polytropic strains, such as JHM and A59, primarily cause hepatitis, enteritis, and encephalitis. Murine coronavirus is an important pathogen in the laboratory mouse and the laboratory rat. It is the most studied coronavirus in animals other than humans, and has been used as an animal disease model for many virological and clinical studies.
Seoul orthohantavirus (SEOV) is a member of the genus Orthohantavirus of rodent-borne viruses, and is one of the four hantaviruses that are known to cause Hantavirus hemorrhagic fever with renal syndrome (HFRS). It is an Old World hantavirus; a negative sense, single-stranded, tri-segmented RNA virus.

Argentine hemorrhagic fever (AHF) or O'Higgins disease, also known in Argentina as mal de los rastrojos is a hemorrhagic fever and zoonotic infectious disease occurring in Argentina. It is caused by the Junín virus. Its reservoir of infection is the drylands vesper mouse, a rodent found in Argentina and Paraguay.

Argentinian mammarenavirus, better known as the Junin virus or Junín virus (JUNV), is an arenavirus in the Mammarenavirus genus that causes Argentine hemorrhagic fever (AHF). The virus took its original name from the city of Junín, around which the first cases of infection were reported, in 1958.

Chapare mammarenavirus or Chapare virus is a virus from the family Arenaviridae which causes a hemorrhagic fever in humans known as Chapare hemorrhagic fever. It was first described after an outbreak of a novel zoonotic mammarenavirus infection occurred in the village of Samuzabeti, Chapare Province, Bolivia, in January 2003. A small number of people were infected and one person died.

Lisa Ellen Hensley is the associate director of science at the Office of the Chief Scientist, National Institute of Allergy and Infectious Disease Integrated Research Facility in Frederick, Maryland. She was previously a civilian microbiologist in the virology division of the United States Army Medical Research Institute of Infectious Diseases (USAMRIID). Hensley is one of the premier researchers of some of the world's most dangerous infections, including Ebola hemorrhagic fever, Lassa fever, the coronavirus diseases Severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), and smallpox. She has been involved in research uncovering critical mechanisms in the pathogenesis of hemorrhagic fever viruses, and has used those discoveries to develop candidate therapeutic drugs for their treatment.
Brazilian hemorrhagic fever (BzHF) is an infectious disease caused by Brazilian mammarenavirus, an arenavirus. Brazilian mammarenavirus is one of the arenaviruses from South America to cause hemorrhagic fever. It shares a common progenitor with Argentinian mammarenavirus, Machupo mammarenavirus, Tacaribe mammarenavirus, and Guanarito mammarenavirus. It is an enveloped RNA virus and is highly infectious and lethal. Very little is known about this disease, but it is thought to be transmitted by the excreta of rodents. This virus has also been implicated as a means for bioterrorism, as it can be spread through aerosols.
Mayaro virus disease is a mosquito-borne zoonotic pathogen endemic to certain humid forests of tropical South America. Infection with Mayaro virus causes an acute, self-limited dengue-like illness of 3–5 days' duration. The causative virus, abbreviated MAYV, is in the family Togaviridae, and genus Alphavirus. It is closely related to other alphaviruses that produce a dengue-like illness accompanied by long-lasting arthralgia. It is only known to circulate in tropical South America.

Marburg virus (MARV) is a hemorrhagic fever virus of the Filoviridae family of viruses and a member of the species Marburg marburgvirus, genus Marburgvirus. It causes Marburg virus disease in primates, a form of viral hemorrhagic fever. The virus is considered to be extremely dangerous. The World Health Organization (WHO) rates it as a Risk Group 4 Pathogen. In the United States, the National Institute of Allergy and Infectious Diseases ranks it as a Category A Priority Pathogen and the Centers for Disease Control and Prevention lists it as a Category A Bioterrorism Agent. It is also listed as a biological agent for export control by the Australia Group.

Zaire ebolavirus, more commonly known as Ebola virus, is one of six known species within the genus Ebolavirus. Four of the six known ebolaviruses, including EBOV, cause a severe and often fatal hemorrhagic fever in humans and other mammals, known as Ebola virus disease (EVD). Ebola virus has caused the majority of human deaths from EVD, and was the cause of the 2013–2016 epidemic in western Africa, which resulted in at least 28,646 suspected cases and 11,323 confirmed deaths.
Batai orthobunyavirus (BATV) is a RNA virus belonging to order Bunyavirales, genus Orthobunyavirus.












