
Rift Valley fever (RVF) is a viral disease of humans and livestock that can cause mild to severe symptoms. The mild symptoms may include: fever, muscle pains, and headaches which often last for up to a week. The severe symptoms may include: loss of sight beginning three weeks after the infection, infections of the brain causing severe headaches and confusion, and bleeding together with liver problems which may occur within the first few days. Those who have bleeding have a chance of death as high as 50%.
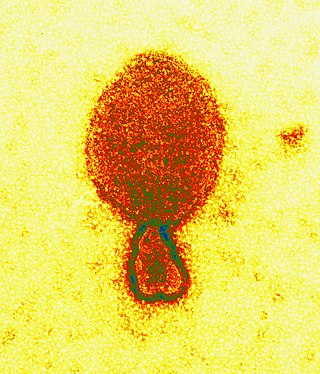
Henipavirus is a genus of negative-strand RNA viruses in the family Paramyxoviridae, order Mononegavirales containing six established species, and numerous others still under study. Henipaviruses are naturally harboured by several species of small mammals, notably pteropid fruit bats, microbats of several species, and shrews. Henipaviruses are characterised by long genomes and a wide host range. Their recent emergence as zoonotic pathogens capable of causing illness and death in domestic animals and humans is a cause of concern.
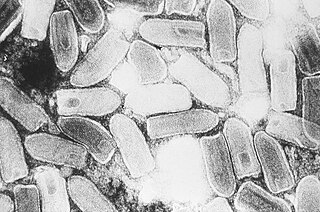
Indiana vesiculovirus, formerly Vesicular stomatitis Indiana virus is a virus in the family Rhabdoviridae; the well-known Rabies lyssavirus belongs to the same family. VSIV can infect insects, cattle, horses and pigs. It has particular importance to farmers in certain regions of the world where it infects cattle. This is because its clinical presentation is identical to the very important foot and mouth disease virus.

Rhabdoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates, invertebrates, plants, fungi and protozoans serve as natural hosts. Diseases associated with member viruses include rabies encephalitis caused by the rabies virus, and flu-like symptoms in humans caused by vesiculoviruses. The name is derived from Ancient Greek rhabdos, meaning rod, referring to the shape of the viral particles. The family has 40 genera, most assigned to three subfamilies.
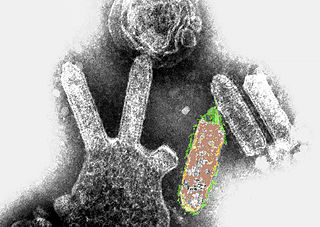
Lyssavirus is a genus of RNA viruses in the family Rhabdoviridae, order Mononegavirales. Mammals, including humans, can serve as natural hosts. The genus Lyssavirus includes the rabies virus traditionally associated with the disease of the same name.
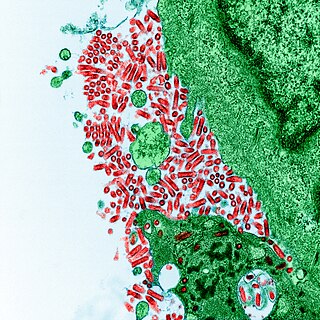
Rabies virus, scientific name Rabies lyssavirus, is a neurotropic virus that causes rabies in animals, including humans. Rabies transmission can occur through the saliva of animals and less commonly through contact with human saliva. Rabies lyssavirus, like many rhabdoviruses, has an extremely wide host range. In the wild it has been found infecting many mammalian species, while in the laboratory it has been found that birds can be infected, as well as cell cultures from mammals, birds, reptiles and insects. Rabies is reported in more than 150 countries and on all continents except Antarctica. The main burden of disease is reported in Asia and Africa, but some cases have been reported also in Europe in the past 10 years, especially in returning travellers.
Chandipura vesiculovirus (CHPV) is a member of the Rhabdoviridae family that is associated with an encephalitic illness in humans. It was first identified in 1965 after isolation from the blood of two patients from Chandipura village in Maharashtra state, India and has been associated with a number of otherwise unexplained outbreaks of encephalitic illness in central India. The most recent occurred in Andhra Pradesh and Maharashtra in June–August 2003 with 329 children affected and 183 deaths. Further sporadic cases and deaths in children were observed in Gujarat state in 2004.

Australian bat lyssavirus (ABLV), originally named Pteropid lyssavirus (PLV), is a enzootic virus closely related to the rabies virus. It was first identified in a 5-month-old juvenile black flying fox collected near Ballina in northern New South Wales, Australia, in January 1995 during a national surveillance program for the recently identified Hendra virus. ABLV is the seventh member of the genus Lyssavirus and the only Lyssavirus member present in Australia. ABLV has been categorized to the Phylogroup I of the Lyssaviruses.
Lagos bat lyssavirus, formerly Lagos bat virus (LBV) is a Lyssavirus of southern and central Africa that causes a rabies-like illness in mammals. It was first isolated from a fruit bat from Lagos Island, Nigeria in 1956. Brain samples from the bat showed poor cross-reactivity to rabies antibodies but the virus was found to be closely related to the rabies virus. This was the first discovery of a rabies-related virus. Until this time, rabies was thought to have a single causal agent.
Duvenhage lyssavirus (DUVV) is a member of the genus Lyssavirus, which also contains the rabies virus. The virus was discovered in 1970, when a South African farmer died of a rabies-like encephalitic illness, after being bitten by a bat. In 2006, Duvenhage virus killed a second person, when a man was scratched by a bat in North West Province, South Africa, 80 km from the 1970 infection. He developed a rabies-like illness 27 days after the bat encounter, and died 14 days after the onset of illness. A 34-year-old woman who died in Amsterdam on December 8, 2007, was the third recorded fatality. She had been scratched on the nose by a small bat while travelling through Kenya in October 2007, and was admitted to hospital four weeks later with rabies-like symptoms.
Seoul orthohantavirus (SEOV) is a member of the genus Orthohantavirus of rodent-borne viruses, and is one of the four hantaviruses that are known to cause Hantavirus hemorrhagic fever with renal syndrome (HFRS). It is an Old World hantavirus; a negative sense, single-stranded, tri-segmented RNA virus.

Veterinary virology is the study of viruses in non-human animals. It is an important branch of veterinary medicine.

Rabies is a viral disease that causes encephalitis in humans and other mammals. It was historically referred to as hydrophobia due to the symptom of panic when presented with liquids to drink. Early symptoms can include fever and abnormal sensations at the site of exposure. These symptoms are followed by one or more of the following symptoms: nausea, vomiting, violent movements, uncontrolled excitement, fear of water, an inability to move parts of the body, confusion, and loss of consciousness. Once symptoms appear, the result is virtually always death, regardless of treatment. The time period between contracting the disease and the start of symptoms is usually one to three months but can vary from less than one week to more than one year. The time depends on the distance the virus must travel along peripheral nerves to reach the central nervous system.

A virus is a submicroscopic infectious agent that replicates only inside the living cells of an organism. Viruses infect all life forms, from animals and plants to microorganisms, including bacteria and archaea. Viruses are found in almost every ecosystem on Earth and are the most numerous type of biological entity. Since Dmitri Ivanovsky's 1892 article describing a non-bacterial pathogen infecting tobacco plants and the discovery of the tobacco mosaic virus by Martinus Beijerinck in 1898, more than 11,000 of the millions of virus species have been described in detail. The study of viruses is known as virology, a subspeciality of microbiology.

In animals, rabies is a viral zoonotic neuroinvasive disease which causes inflammation in the brain and is usually fatal. Rabies, caused by the rabies virus, primarily infects mammals. In the laboratory it has been found that birds can be infected, as well as cell cultures from birds, reptiles and insects. The brains of animals with rabies deteriorate. As a result, they tend to behave bizarrely and often aggressively, increasing the chances that they will bite another animal or a person and transmit the disease.

Arctic rabies virus is a strain of Rabies lyssavirus that circulates throughout the arctic regions of Alaska, Canada, Greenland, and Russia. There have been no cases in Sweden or mainland Norway in over 100 years. The virus is, however, found on Svalbard. No cases have been reported in Finland since 1989. The Arctic fox is the main host.

The bat virome is the group of viruses associated with bats. Bats host a diverse array of viruses, including all seven types described by the Baltimore classification system: (I) double-stranded DNA viruses; (II) single-stranded DNA viruses; (III) double-stranded RNA viruses; (IV) positive-sense single-stranded RNA viruses; (V) negative-sense single-stranded RNA viruses; (VI) positive-sense single-stranded RNA viruses that replicate through a DNA intermediate; and (VII) double-stranded DNA viruses that replicate through a single-stranded RNA intermediate. The greatest share of bat-associated viruses identified as of 2020 are of type IV, in the family Coronaviridae.
European bat 1 lyssavirus(EBLV-1) is one of three rabies virus-like agents of the genus Lyssavirus found in serotine bats in Spain. Strains of EBLV-1 have been identified as EBLV-1a and EBLV-1b. EBLV-1a was isolated from bats found in the Netherlands and Russia, while EBLV-1b was found in bats in France, the Netherlands and Iberia. E. isabellinus bats are the EBLV-1b reservoir in the Iberian Peninsula. Between 1977 and 2010, 959 bat rabies cases of EBLV-1 were reported to the World Health Organization (WHO) Rabies Bulletin.
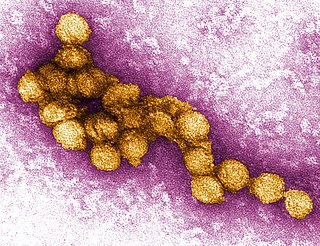
West Nile virus (WNV) is a single-stranded RNA virus that causes West Nile fever. It is a member of the family Flaviviridae, from the genus Flavivirus, which also contains the Zika virus, dengue virus, and yellow fever virus. The virus is primarily transmitted by mosquitoes, mostly species of Culex. The primary hosts of WNV are birds, so that the virus remains within a "bird–mosquito–bird" transmission cycle. The virus is genetically related to the Japanese encephalitis family of viruses. Humans and horses both exhibit disease symptoms from the virus, and symptoms rarely occur in other animals.
West Caucasian bat lyssavirus (WCBL) is a member of genus Lyssavirus, family Rhabdoviridae and order Mononegavirales. This virus was first isolated from Miniopterus schreibersii, in the western Caucasus Mountains of southeastern Europe in 2002. WCBL is the most divergent form of Lyssavirus, and is found in Miniopterus bats (insectivorous), Rousettus aegyptiacus, and Eidolon helvum. The latter two are both fruit bats. The virus is fragile as it can be inactivated by UV light and chemicals, such as ether, chloroform, and bleach. WCBL has not been known to infect humans thus far.













