
In organic chemistry, a ketone is a functional group with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.
Pyrimidine is an aromatic, heterocyclic, organic compound similar to pyridine. One of the three diazines, it has nitrogen atoms at positions 1 and 3 in the ring. The other diazines are pyrazine and pyridazine.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.

In organic chemistry, a cyanohydrin or hydroxynitrile is a functional group found in organic compounds in which a cyano and a hydroxy group are attached to the same carbon atom. The general formula is R2C(OH)CN, where R is H, alkyl, or aryl. Cyanohydrins are industrially important precursors to carboxylic acids and some amino acids. Cyanohydrins can be formed by the cyanohydrin reaction, which involves treating a ketone or an aldehyde with hydrogen cyanide (HCN) in the presence of excess amounts of sodium cyanide (NaCN) as a catalyst:

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula C
14H
8O
2. Isomers include various quinone derivatives. The term anthraquinone however refers to the isomer, 9,10-anthraquinone wherein the keto groups are located on the central ring. It is a building block of many dyes and is used in bleaching pulp for papermaking. It is a yellow, highly crystalline solid, poorly soluble in water but soluble in hot organic solvents. It is almost completely insoluble in ethanol near room temperature but 2.25 g will dissolve in 100 g of boiling ethanol. It is found in nature as the rare mineral hoelite.

Anisole, or methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances. The compound is mainly made synthetically and is a precursor to other synthetic compounds. Structurally, it is an ether with a methyl and phenyl group attached. Anisole is a standard reagent of both practical and pedagogical value.

Acetone, is an organic compound with the formula (CH3)2CO. It is the simplest and smallest ketone. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor.

Dibenzylideneacetone or dibenzalacetone, often abbreviated dba, is an organic compound with the formula C17H14O. It is a pale-yellow solid insoluble in water, but soluble in ethanol.

Nitrosobenzene is the organic compound with the formula C6H5NO. It is one of the prototypical organic nitroso compounds. Characteristic of its functional group, it is a dark green species that exists in equilibrium with its pale yellow dimer. Both monomer and dimer are diamagnetic.
The Gattermann reaction, (also known as the Gattermann formylation and the Gattermann salicylaldehyde synthesis) is a chemical reaction in which aromatic compounds are formylated by a mixture of hydrogen cyanide (HCN) and hydrogen chloride (HCl) in the presence of a Lewis acid catalyst such as AlCl3. It is named for the German chemist Ludwig Gattermann and is similar to the Friedel–Crafts reaction.
The Blanc chloromethylation is the chemical reaction of aromatic rings with formaldehyde and hydrogen chloride to form chloromethyl arenes. The reaction is catalyzed by Lewis acids such as zinc chloride. The reaction was discovered by Gustave Louis Blanc (1872-1927) in 1923
Acetone cyanohydrin (ACH) is an organic compound used in the production of methyl methacrylate, the monomer of the transparent plastic polymethyl methacrylate (PMMA), also known as acrylic. It liberates hydrogen cyanide easily, so it is used as a source of such. For this reason, this cyanohydrin is also highly toxic.

Durene, or 1,2,4,5-tetramethylbenzene, is an organic compound with the formula C6H2(CH3)4. It is a colourless solid with a sweet odor. The compound is classified as an alkylbenzene. It is one of three isomers of tetramethylbenzene, the other two being prehnitene (1,2,3,4-tetramethylbenzene) and isodurene (1,2,3,5-tetramethylbenzene). Durene has an unusually high melting point (79.2 °C), reflecting its high molecular symmetry.
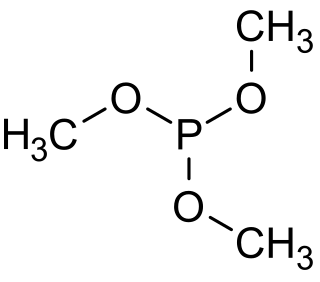
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is the simplest phosphite ester and finds used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.

(Mesitylene)molybdenum tricarbonyl is an organomolybdenum compound derived from the aromatic compound mesitylene (1,3,5-trimethylbenzene) and molybdenum carbonyl. It exists as pale yellow crystals, which are soluble in organic solvents but decompose when in solution. It has been examined as a catalyst and reagent.
4-Nitrotoluene or para-nitrotoluene is an organic compound with the formula CH3C6H4NO2. It is a pale yellow solid. It is one of three isomers of nitrotoluene.

Isodurene or 1,2,3,5-tetramethylbenzene is an organic compound with the formula C6H2(CH3)4, classified as an aromatic hydrocarbon. It is a flammable colorless liquid which is nearly insoluble in water but soluble in organic solvents. It occurs naturally in coal tar. Isodurene is one of three isomers of tetramethylbenzene, the other two being prehnitene (1,2,3,4-tetramethylbenzene) and durene (1,2,4,5-tetramethylbenzene).

In organic chemistry, a benzylidene acetal is the functional group with the structural formula C6H5CH(OR)2 (R = alkyl, aryl). Benzylidene acetals are used as protecting groups in glycochemistry. These compounds can also be oxidized to carboxylic acids in order to open important biological molecules, such as glycosaminoglycans, to other routes of synthesis. They arise from the reaction of a 1,2- or 1,3-diols with benzaldehyde. Other aromatic aldehydes are also used.
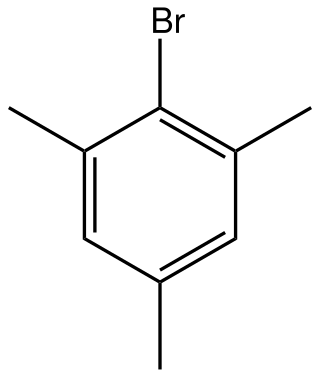
Mesityl bromide is an organic compound with the formula (CH3)3C6H2Br. It is a derivative of mesitylene (1,3,5-trimethylbenzene) with one ring H replaced by Br. The compound is a colorless oil. It is a standard electron-rich aryl halide substrate for cross coupling reactions. With magnesium it reacts to give the Grignard reagent, which is used in the preparation of tetramesityldiiron.


















