
Dementia with Lewy bodies (DLB) is a type of dementia characterized by changes in sleep, behavior, cognition, movement, and regulation of automatic bodily functions. Memory loss is not always an early symptom. The disease worsens over time and is usually diagnosed when cognitive impairment interferes with normal daily functioning. Together with Parkinson's disease dementia, DLB is one of the two Lewy body dementias. It is a common form of dementia, but the prevalence is not known accurately and many diagnoses are missed. The disease was first described by Kenji Kosaka in 1976.

Binswanger's disease, also known as subcortical leukoencephalopathy and subcortical arteriosclerotic encephalopathy, is a form of small-vessel vascular dementia caused by damage to the white brain matter. White matter atrophy can be caused by many circumstances including chronic hypertension as well as old age. This disease is characterized by loss of memory and intellectual function and by changes in mood. These changes encompass what are known as executive functions of the brain. It usually presents between 54 and 66 years of age, and the first symptoms are usually mental deterioration or stroke.

In neuroanatomy, the precuneus is the portion of the superior parietal lobule on the medial surface of each brain hemisphere. It is located in front of the cuneus. The precuneus is bounded in front by the marginal branch of the cingulate sulcus, at the rear by the parieto-occipital sulcus, and underneath by the subparietal sulcus. It is involved with episodic memory, visuospatial processing, reflections upon self, and aspects of consciousness.

The occipital lobe is one of the four major lobes of the cerebral cortex in the brain of mammals. The name derives from its position at the back of the head, from the Latin ob, 'behind', and caput, 'head'.

Frontotemporal dementia (FTD), frontotemporal degeneration disease, or frontotemporal neurocognitive disorder, encompasses several types of dementia involving the progressive degeneration of the brain's frontal and temporal lobes. FTDs broadly present as behavioral or language disorders with gradual onsets. Common signs and symptoms include significant changes in social and personal behavior, apathy, blunting of emotions, and deficits in both expressive and receptive language. Signs and symptoms tend to appear in late adulthood, typically between the ages of 45 and 65. Men and women appear to be equally affected. FTD is the second most prevalent type of early onset dementia after Alzheimer's disease. Currently, there is no cure, but there are treatments that help alleviate symptoms.
Cerebral atrophy is a common feature of many of the diseases that affect the brain. Atrophy of any tissue means a decrement in the size of the cell, which can be due to progressive loss of cytoplasmic proteins. In brain tissue, atrophy describes a loss of neurons and the connections between them. Brain atrophy can be classified into two main categories: generalized and focal atrophy. Generalized atrophy occurs across the entire brain whereas focal atrophy affects cells in a specific location. If the cerebral hemispheres are affected, conscious thought and voluntary processes may be impaired.
Semantic dementia (SD), also known as semantic variant primary progressive aphasia (svPPA), is a progressive neurodegenerative disorder characterized by loss of semantic memory in both the verbal and non-verbal domains. However, the most common presenting symptoms are in the verbal domain. Semantic dementia is a disorder of semantic memory that causes patients to lose the ability to match words or images to their meanings. However, it is fairly rare for patients with semantic dementia to develop category specific impairments, though there have been documented cases of it occurring. Typically, a more generalized semantic impairment results from dimmed semantic representations in the brain.
Imaging genetics refers to the use of anatomical or physiological imaging technologies as phenotypic assays to evaluate genetic variation. Scientists that first used the term imaging genetics were interested in how genes influence psychopathology and used functional neuroimaging to investigate genes that are expressed in the brain.

Corticobasal degeneration (CBD) is a rare neurodegenerative disease involving the cerebral cortex and the basal ganglia. CBD symptoms typically begin in people from 50 to 70 years of age, and typical survival before death is eight years. It is characterized by marked disorders in movement and cognition, and is classified as one of the Parkinson plus syndromes. Diagnosis is difficult, as symptoms are often similar to those of other disorders, such as Parkinson's disease, progressive supranuclear palsy, and dementia with Lewy bodies, and a definitive diagnosis of CBD can only be made upon neuropathologic examination.
Cortical blindness is the total or partial loss of vision in a normal-appearing eye caused by damage to the brain's occipital cortex. Cortical blindness can be acquired or congenital, and may also be transient in certain instances. Acquired cortical blindness is most often caused by loss of blood flow to the occipital cortex from either unilateral or bilateral posterior cerebral artery blockage and by cardiac surgery. In most cases, the complete loss of vision is not permanent and the patient may recover some of their vision. Congenital cortical blindness is most often caused by perinatal ischemic stroke, encephalitis, and meningitis. Rarely, a patient with acquired cortical blindness may have little or no insight that they have lost vision, a phenomenon known as Anton–Babinski syndrome.
The posterior cerebral artery (PCA) is one of a pair of cerebral arteries that supply oxygenated blood to the occipital lobe, part of the back of the human brain. The two arteries originate from the distal end of the basilar artery, where it bifurcates into the left and right posterior cerebral arteries. These anastomose with the middle cerebral arteries and internal carotid arteries via the posterior communicating arteries.
Psychoorganic syndrome (POS), also known as organic psychosyndrome, is a progressive disease comparable to presenile dementia. It consists of psychopathological complex of symptoms that are caused by organic brain disorders that involve a reduction in memory and intellect. Psychoorganic syndrome is often accompanied by asthenia.

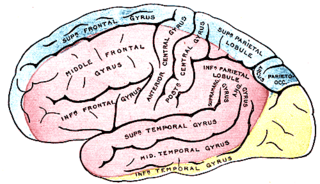
The inferior temporal gyrus is one of three gyri of the temporal lobe and is located below the middle temporal gyrus, connected behind with the inferior occipital gyrus; it also extends around the infero-lateral border on to the inferior surface of the temporal lobe, where it is limited by the inferior sulcus. This region is one of the higher levels of the ventral stream of visual processing, associated with the representation of objects, places, faces, and colors. It may also be involved in face perception, and in the recognition of numbers and words.

The posterior cingulate cortex (PCC) is the caudal part of the cingulate cortex, located posterior to the anterior cingulate cortex. This is the upper part of the "limbic lobe". The cingulate cortex is made up of an area around the midline of the brain. Surrounding areas include the retrosplenial cortex and the precuneus.

Posterior cerebral artery syndrome is a condition whereby the blood supply from the posterior cerebral artery (PCA) is restricted, leading to a reduction of the function of the portions of the brain supplied by that vessel: the occipital lobe, the inferomedial temporal lobe, a large portion of the thalamus, and the upper brainstem and midbrain.
Phonagnosia is a type of agnosia, or loss of knowledge, that involves a disturbance in the recognition of familiar voices and the impairment of voice discrimination abilities in which the affected individual does not suffer from comprehension deficits. Phonagnosia is an auditory agnosia, an acquired auditory processing disorder resulting from brain damage, other auditory agnosias include cortical deafness and auditory verbal agnosia also known as pure word deafness.
Alzheimer's Disease Neuroimaging Initiative (ADNI) is a multisite study that aims to improve clinical trials for the prevention and treatment of Alzheimer's disease (AD). This cooperative study combines expertise and funding from the private and public sector to study subjects with AD, as well as those who may develop AD and controls with no signs of cognitive impairment. Researchers at 63 sites in the US and Canada track the progression of AD in the human brain with neuroimaging, biochemical, and genetic biological markers. This knowledge helps to find better clinical trials for the prevention and treatment of AD. ADNI has made a global impact, firstly by developing a set of standardized protocols to allow the comparison of results from multiple centers, and secondly by its data-sharing policy which makes available all at the data without embargo to qualified researchers worldwide. To date, over 1000 scientific publications have used ADNI data. A number of other initiatives related to AD and other diseases have been designed and implemented using ADNI as a model. ADNI has been running since 2004 and is currently funded until 2021.

Ulegyria is a diagnosis used to describe a specific type of cortical scarring in the deep regions of the sulcus that leads to distortion of the gyri. Ulegyria is identified by its characteristic "mushroom-shaped" gyri, in which scarring causes shrinkage and atrophy in the deep sulcal regions while the surface gyri are spared. This condition is most often caused by hypoxic-ischemic brain injury in the perinatal period. The effects of ulegyria can range in severity, although it is most commonly associated with cerebral palsy, mental retardation and epilepsy. N.C. Bresler was the first to view ulegyria in 1899 and described this abnormal morphology in the brain as “mushroom-gyri." Although ulegyria was first identified in 1899, there is still limited information known or reported about the condition.
Parkinson's disease dementia (PDD) is dementia that is associated with Parkinson's disease (PD). Together with dementia with Lewy bodies (DLB), it is one of the Lewy body dementias characterized by abnormal deposits of Lewy bodies in the brain.
The occipital face area (OFA) is a region of the human cerebral cortex which is specialised for face perception. The OFA is located on the lateral surface of the occipital lobe adjacent to the inferior occipital gyrus. The OFA comprises a network of brain regions including the fusiform face area (FFA) and posterior superior temporal sulcus (STS) which support facial processing.











