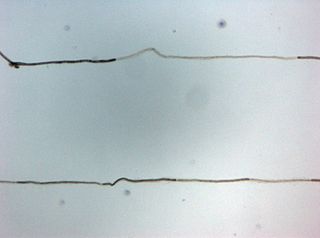
Myelin is a lipid-rich material that surrounds nerve cell axons to insulate them and increase the rate at which electrical impulses pass along the axon. The myelinated axon can be likened to an electrical wire with insulating material (myelin) around it. However, unlike the plastic covering on an electrical wire, myelin does not form a single long sheath over the entire length of the axon. Rather, myelin ensheaths the axon segmentally: in general, each axon is encased in multiple long sheaths with short gaps between, called nodes of Ranvier. At the nodes of Ranvier, which are approximately one thousandth of a mm in length, the axon's membrane is bare of myelin.
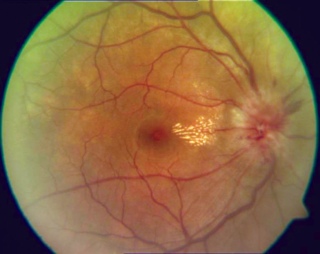
Optic neuritis describes any condition that causes inflammation of the optic nerve; it may be associated with demyelinating diseases, or infectious or inflammatory processes.
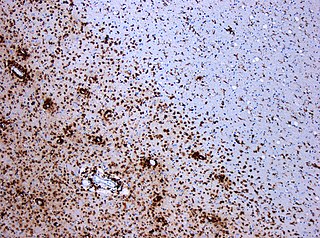
Multiple sclerosis (MS) is an autoimmune disease in which the insulating covers of nerve cells in the brain and spinal cord are damaged. This damage disrupts the ability of parts of the nervous system to transmit signals, resulting in a range of signs and symptoms, including physical, mental, and sometimes psychiatric problems. Specific symptoms can include double vision, vision loss, eye pain, muscle weakness, and loss of sensation or coordination. MS takes several forms, with new symptoms either occurring in isolated attacks or building up over time. In the relapsing forms of MS, between attacks, symptoms may disappear completely, although some permanent neurological problems often remain, especially as the disease advances. In the progressive forms of MS, bodily function slowly deteriorates and disability worsens once symptoms manifest and will steadily continue to do so if the disease is left untreated.
Myelitis is inflammation of the spinal cord which can disrupt the normal responses from the brain to the rest of the body, and from the rest of the body to the brain. Inflammation in the spinal cord can cause the myelin and axon to be damaged resulting in symptoms such as paralysis and sensory loss. Myelitis is classified to several categories depending on the area or the cause of the lesion; however, any inflammatory attack on the spinal cord is often referred to as transverse myelitis.

Oligodendrocytes, also known as oligodendroglia, are a type of neuroglia whose main functions are to provide support and insulation to axons within the central nervous system (CNS) of jawed vertebrates. Their function is similar to that of Schwann cells, which perform the same task in the peripheral nervous system (PNS). Oligodendrocytes accomplish this by forming the myelin sheath around axons. Unlike Schwann cells, a single oligodendrocyte can extend its processes to cover around 50 axons, with each axon being wrapped in approximately 1 μm of myelin sheath. Furthermore, an oligodendrocyte can provide myelin segments for multiple adjacent axons.
Oligodendrocyte progenitor cells (OPCs), also known as oligodendrocyte precursor cells, NG2-glia, O2A cells, or polydendrocytes, are a subtype of glia in the central nervous system named for their essential role as precursors to oligodendrocytes. They are typically identified in the human by co-expression of PDGFRA and CSPG4.
Experimental autoimmune encephalomyelitis, sometimes experimental allergic encephalomyelitis (EAE), is an animal model of brain inflammation. It is an inflammatory demyelinating disease of the central nervous system (CNS). It is mostly used with rodents and is widely studied as an animal model of the human CNS demyelinating diseases, including multiple sclerosis (MS) and acute disseminated encephalomyelitis (ADEM). EAE is also the prototype for T-cell-mediated autoimmune disease in general.
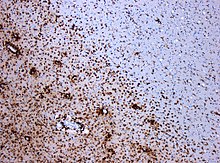
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an acquired autoimmune disease of the peripheral nervous system characterized by progressive weakness and impaired sensory function in the legs and arms. The disorder is sometimes called chronic relapsing polyneuropathy (CRP) or chronic inflammatory demyelinating polyradiculoneuropathy. CIDP is closely related to Guillain–Barré syndrome and it is considered the chronic counterpart of that acute disease. Its symptoms are also similar to progressive inflammatory neuropathy. It is one of several types of neuropathy.

Multiple sclerosis is an inflammatory demyelinating disease of the CNS in which activated immune cells invade the central nervous system and cause inflammation, neurodegeneration, and tissue damage. The underlying cause is currently unknown. Current research in neuropathology, neuroimmunology, neurobiology, and neuroimaging, together with clinical neurology, provide support for the notion that MS is not a single disease but rather a spectrum.
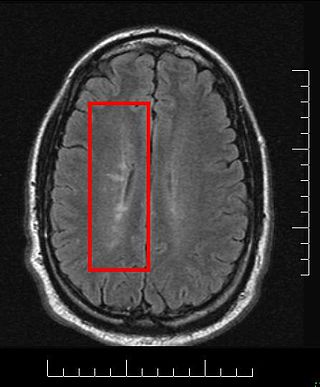
Multiple sclerosis and other demyelinating diseases of the central nervous system (CNS) produce lesions and glial scars or scleroses. They present different shapes and histological findings according to the underlying condition that produces them.
Remyelination is the process of propagating oligodendrocyte precursor cells to form oligodendrocytes to create new myelin sheaths on demyelinated axons in the CNS. This is a process naturally regulated in the body and tends to be very efficient in a healthy CNS. The process creates a thinner myelin sheath than normal, but it helps to protect the axon from further damage, from overall degeneration, and proves to increase conductance once again. The processes underlying remyelination are under investigation in the hope of finding treatments for demyelinating diseases, such as multiple sclerosis.
Inflammatory demyelinating diseases (IDDs), sometimes called Idiopathic (IIDDs) due to the unknown etiology of some of them, are a heterogenous group of demyelinating diseases - conditions that cause damage to myelin, the protective sheath of nerve fibers - that occur against the background of an acute or chronic inflammatory process. IDDs share characteristics with and are often grouped together under Multiple Sclerosis. They are sometimes considered different diseases from Multiple Sclerosis, but considered by others to form a spectrum differing only in terms of chronicity, severity, and clinical course.

Baló's concentric sclerosis is a disease in which the white matter of the brain appears damaged in concentric layers, leaving the axis cylinder intact. It was described by József Mátyás Baló who initially named it "leuko-encephalitis periaxialis concentrica" from the previous definition, and it is currently considered one of the borderline forms of multiple sclerosis.
Myelinogenesis is the formation and development of myelin sheaths in the nervous system, typically initiated in late prenatal neurodevelopment and continuing throughout postnatal development. Myelinogenesis continues throughout the lifespan to support learning and memory via neural circuit plasticity as well as remyelination following injury. Successful myelination of axons increases action potential speed by enabling saltatory conduction, which is essential for timely signal conduction between spatially separate brain regions, as well as provides metabolic support to neurons.

Gap junction beta-1 protein (GJB1), also known as connexin 32 (Cx32), is a transmembrane protein that in humans is encoded by the GJB1 gene. Gap junction beta-1 protein is a member of the gap junction connexin family of proteins that regulates and controls the transfer of communication signals across cell membranes, primarily in the liver and peripheral nervous system. However, the protein is expressed in multiple organs, including in oligodendrocytes in the central nervous system.

Tumefactive multiple sclerosis is a condition in which the central nervous system of a person has multiple demyelinating lesions with atypical characteristics for those of standard multiple sclerosis (MS). It is called tumefactive as the lesions are "tumor-like" and they mimic tumors clinically, radiologically and sometimes pathologically.
Anti-MAG peripheral neuropathy is a specific type of peripheral neuropathy in which the person's own immune system attacks cells that are specific in maintaining a healthy nervous system. As these cells are destroyed by antibodies, the nerve cells in the surrounding region begin to lose function and create many problems in both sensory and motor function. Specifically, antibodies against myelin-associated glycoprotein (MAG) damage Schwann cells. While the disorder occurs in only 10% of those afflicted with peripheral neuropathy, people afflicted have symptoms such as muscle weakness, sensory problems, and other motor deficits usually starting in the form of a tremor of the hands or trouble walking. There are, however, multiple treatments that range from simple exercises in order to build strength to targeted drug treatments that have been shown to improve function in people with this type of peripheral neuropathy.

Multiple sclerosis (MS) can be pathologically defined as the presence of distributed glial scars (scleroses) in the central nervous system that must show dissemination in time (DIT) and in space (DIS) to be considered MS lesions.
MOG antibody disease (MOGAD) or MOG antibody-associated encephalomyelitis (MOG-EM) is an inflammatory demyelinating disease of the central nervous system. Serum anti-myelin oligodendrocyte glycoprotein antibodies are present in up to half of patients with an acquired demyelinating syndrome and have been described in association with a range of phenotypic presentations, including acute disseminated encephalomyelitis, optic neuritis, transverse myelitis, and neuromyelitis optica.
Anti-neurofascin demyelinating diseases refers to health conditions engendered by auto-antibodies against neurofascins, which can produce both central and peripheral demyelination. Some cases of combined central and peripheral demyelination (CCPD) could be produced by them.