
Mescaline or mescalin (3,4,5-trimethoxyphenethylamine) is a naturally occurring psychedelic protoalkaloid of the substituted phenethylamine class, known for its hallucinogenic effects comparable to those of LSD and psilocybin.

Gallic acid (also known as 3,4,5-trihydroxybenzoic acid) is a trihydroxybenzoic acid with the formula C6H2(OH)3CO2H. It is classified as a phenolic acid. It is found in gallnuts, sumac, witch hazel, tea leaves, oak bark, and other plants. It is a white solid, although samples are typically brown owing to partial oxidation. Salts and esters of gallic acid are termed "gallates".
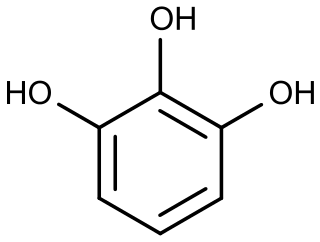
Phloroglucinol is an organic compound with the formula C6H3(OH)3. It is a colorless solid. It is used in the synthesis of pharmaceuticals and explosives. Phloroglucinol is one of three isomeric benzenetriols. The other two isomers are hydroxyquinol (1,2,4-benzenetriol) and pyrogallol (1,2,3-benzenetriol). Phloroglucinol, and its benzenetriol isomers, are still defined as "phenols" according to the IUPAC official nomenclature rules of chemical compounds. Many such monophenolics are often termed polyphenols.
Pyrogallol is an organic compound with the formula C6H3(OH)3. It is a water-soluble, white solid although samples are typically brownish because of its sensitivity toward oxygen. It is one of three isomers of benzenetriols.

Phosphoinositide 3-kinases (PI3Ks), also called phosphatidylinositol 3-kinases, are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which in turn are involved in cancer.

Propyl gallate, or propyl 3,4,5-trihydroxybenzoate is an ester formed by the condensation of gallic acid and propanol. Since 1948, this antioxidant has been added to foods containing oils and fats to prevent oxidation. As a food additive, it is used under the E number E310.
In chemistry, a resorcinarene is a macrocycle, or a cyclic oligomer, based on the condensation of resorcinol (1,3-dihydroxybenzene) and an aldehyde. Resorcinarenes are a type of calixarene. Other types of resorcinarenes include the related pyrogallolarenes and octahydroxypyridines, derived from pyrogallol and 2,6-dihydroxypyridine, respectively.
In enzymology, a phloroglucinol reductase (EC 1.3.1.57) is an enzyme that catalyzes the chemical reaction
In enzymology, a pyrogallol hydroxytransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a pyrogallol 1,2-oxygenase (EC 1.13.11.35) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-hydroxy-1,4-benzoquinone reductase (EC 1.6.5.7) is an enzyme that catalyzes the chemical reaction
The enzyme gallate decarboxylase (EC 4.1.1.59) catalyzes the chemical reaction
The Akt signaling pathway or PI3K-Akt signaling pathway is a signal transduction pathway that promotes survival and growth in response to extracellular signals. Key proteins involved are PI3K and Akt.

The trihydroxybenzenes (or benzenetriols) are organic compounds with the formula C6H3(OH)3. Also classified as polyphenols, they feature three hydroxyl groups substituted onto a benzene ring. They are white solids with modest solubility in water.
Pelobacter acidigallici is the type species in the bacterial genus Pelobacter.
Norbert Pfennig was a German microbiologist.

Tetrahydroxybenzenes or Benzenetetrols are a group of organic compounds which are tetrahydroxy derivatives of benzene. Tetrahydroxybenzene comes in three isomers:
Eubacterium oxidoreducens is a Gram positive bacterium species in the genus Eubacterium.

Dihydrophloroglucinol is a chemical compound found in the pathway of the microbial degradation of phloroglucinol and other phenolic compounds.
Peptoclostridium acidaminophilum is a Gram-positive bacterium species in the family Peptostreptococcaceae, notable for being an amino acid-degrading obligate anaerobe producing or utilizing H2 or formate. It is rod-shaped and motile by a polar to subpolar flagellum. Its type strain is al-2. It produces several relevant enzymes.










