Serotonin–norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant drugs that treat major depressive disorder (MDD), anxiety disorders, obsessive–compulsive disorder (OCD), social phobia, attention-deficit hyperactivity disorder (ADHD), chronic neuropathic pain, fibromyalgia syndrome (FMS), and menopausal symptoms. SNRIs are monoamine reuptake inhibitors; specifically, they inhibit the reuptake of serotonin and norepinephrine. These neurotransmitters are thought to play an important role in mood regulation. SNRIs can be contrasted with the more widely used selective serotonin reuptake inhibitors (SSRIs), which act upon serotonin only.

Medicinal plants, also called medicinal herbs, have been discovered and used in traditional medicine practices since prehistoric times. Plants synthesise hundreds of chemical compounds for functions including defence against insects, fungi, diseases, and herbivorous mammals. Numerous phytochemicals with potential or established biological activity have been identified. However, since a single plant contains widely diverse phytochemicals, the effects of using a whole plant as medicine are uncertain. Further, the phytochemical content and pharmacological actions, if any, of many plants having medicinal potential remain unassessed by rigorous scientific research to define efficacy and safety.

Bazedoxifene, used as bazedoxifene acetate, is a medication for bone problems and possibly for cancer. It is a third-generation selective estrogen receptor modulator (SERM). Since late 2013 it has had U.S. FDA approval for bazedoxifene as part of the combination drug Duavee in the prevention of postmenopausal osteoporosis. It is also being studied for possible treatment of breast cancer and pancreatic cancer.
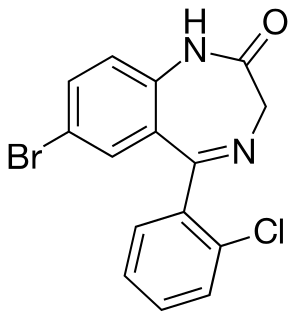
Phenazepam is a benzodiazepine drug, which was developed in the Soviet Union in 1975, and now produced in Russia and some CIS countries.
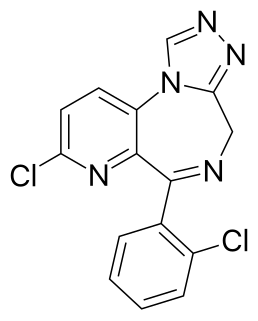
Zapizolam is a pyridodiazepine drug, which is a benzodiazepine analog of pyridotriazolodiazepine group. It has sedative and anxiolytic effects similar to those produced by benzodiazepine derivatives, and has been sold illicitly as a designer drug.

Arfendazam (INN) is a drug which is a benzodiazepine derivative. Arfendazam is a 1,5-benzodiazepine, with the nitrogen atoms located at positions 1 and 5 of the diazepine ring, and so is most closely related to other 1,5-benzodiazepines such as clobazam.
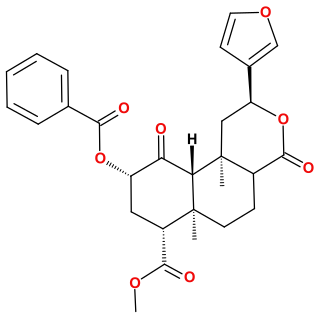
Herkinorin is an opioid analgesic that is an analogue of the natural product salvinorin A. It was discovered in 2005 during structure-activity relationship studies into neoclerodane diterpenes, the family of chemical compounds of which salvinorin A is a member.

Propiram is a partial mu opioid receptor agonist and weak mu antagonist analgesic from the ampromide family of drugs related to other drugs such as phenampromide and diampromide. It was invented in 1963 in the United Kingdom by Bayer but was not widely marketed, although it saw some limited clinical use, especially in dentistry. Propiram reached Phase III clinical trials in the United States and Canada.
2,3-Dihydroxybenzoic acid is a natural phenol found in Phyllanthus acidus and in the aquatic fern Salvinia molesta. It is also abundant in the fruits of Flacourtia inermis. It is a dihydroxybenzoic acid, a type of organic compound. The colorless solid occurs naturally, being formed via the shikimate pathway. It is incorporated into various siderophores, which are molecules that strongly complex iron ions for absorption into bacteria. 2,3-DHB consists of a catechol group, which upon deprotonation binds iron centers very strongly, and the carboxylic acid group by which the ring attaches to various scaffolds through amide bonds. A famous high affinity siderophore is enterochelin, which contains three dihydroxybenzoyl substituents linked to the depsipeptide of serine.
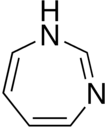
Diazepine is a seven-membered heterocyclic compound with two nitrogen atoms.

Ciclotizolam (WE-973) is a drug which is a thienotriazolodiazepine derivative. It is a partial agonist for the benzodiazepine site of the GABAA receptor, with similar binding affinity to related compounds like brotizolam, but a low efficacy.
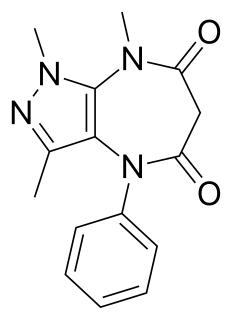
Zomebazam produced by Hoechst is a pyrazolodiazepinone derivative drug with anxiolytic properties. It is structurally related to razobazam and zometapine.

Rhodanine is a 5-membered heterocyclic organic compound possessing a thiazolidine core. It was discovered in 1877 by Marceli Nencki who named it "Rhodaninsaure" in reference to its synthesis from ammonium rhodanide and chloroacetic acid in water.
PSB-SB-487 is a coumarin derivative which is an antagonist at the former orphan receptor GPR55. Unlike older GPR55 antagonists such as O-1918, PSB-SB-487 has good selectivity over the related receptor GPR18, with an IC50 of 113nM at GPR55 vs 12500nM at GPR18. However it has poorer selectivity over other related receptors, acting as a weak antagonist at CB1 with a Ki of 1170nM, and a partial agonist at CB2 with a Ki of 292nM.
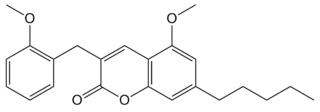
PSB-SB-1202 is a coumarin derivative which is an agonist at the cannabinoid receptors CB1 and CB2, with a CB1 Ki of 32nM and a CB2 Ki of 49nM. It is also a weak antagonist at the related receptor GPR55, with an IC50 of 6350nM, but has no significant affinity for GPR18.
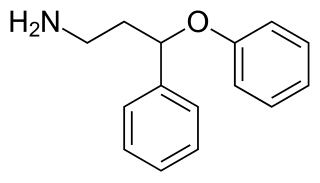
PPPA, or 3-phenoxy-3-phenylpropan-1-amine, is a drug which is described as an antidepressant. It was derived by Eli Lilly from the antihistamine diphenhydramine, a 2-diphenylmethoxyethanamine derivative with additional properties as a selective serotonin reuptake inhibitor (SSRI), and has been the basis for the subsequent discovery of a number of other antidepressant drugs.

Metizolam is a thienotriazolodiazepine that is the demethylated analogue of the closely related etizolam.

The substituted benzofurans are a class of chemical compounds based on the heterocyclyc and polycyclic compound benzofuran. Many medicines use the benzofuran core as a scaffold, but most commonly the term is used to refer to the simpler compounds in this class which include numerous psychoactive drugs, including stimulants, psychedelics and empathogens. In general, these compounds have a benzofuran core to which a 2-aminoethyl group is attached, and combined with a range of other substituents. Some psychoactive derivatives from this family have been sold under the name Benzofury.
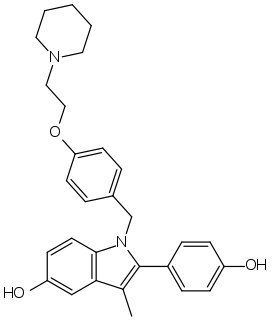
Pipendoxifene (INN) is a nonsteroidal selective estrogen receptor modulator (SERM) that was under development by Ligand Pharmaceuticals and Wyeth-Ayerst Laboratories for the treatment of breast cancer but was not marketed. It is a member of the 2-phenylindole group of SERMs and is structurally related to zindoxifene and the marketed bazedoxifene. The drug reached phase II clinical trials before its development was discontinued. It was synthesized at the same time as bazedoxifene and was intended as a backup drug for bazedoxifene, only to be developed further if bazedoxifene had failed in clinical trials. No further development was reported after 2002 and it was formally announced that development had been terminated in November 2005.

JNJ-28330835 is a drug which acts as a selective androgen receptor modulator (SARM). In studies on rats it was found to enhance muscle growth and sexual behavior but with minimal effects on prostate gland size. A number of related compounds are known, though JNJ-28330835 has progressed furthest through development.