A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae.
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the empirical formula of disulfur dioxide, S2O2. Thus, sulfur monoxide and disulphur dioxide, both compounds of sulphur and oxygen, have the same empirical formula. However, their molecular formulas, which express the number of atoms in each molecule of a chemical compound, are not the same.
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.
In chemistry, the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance in that sample, measured in moles. The molar mass is a bulk, not molecular, property of a substance. The molar mass is an average of many instances of the compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molar mass is appropriate for converting between the mass of a substance and the amount of a substance for bulk quantities.

In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a chemical formula. The informal use of the term formula in science refers to the general construct of a relationship between given quantities.

A dimer is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. The term homodimer is used when the two molecules are identical and heterodimer when they are not. The reverse of dimerisation is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as Bjerrum pairs, after Niels Bjerrum.

Organic peroxides are organic compounds containing the peroxide functional group (ROOR′). If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. Peresters are the peroxy analog of esters and have general structure RC(O)OOR. The O−O bond of peroxides easily breaks, producing free radicals of the form RO•. Thus, organic peroxides are useful as initiators for some types of polymerisation, such as the epoxy resins used in glass-reinforced plastics. MEKP and benzoyl peroxide are commonly used for this purpose. However, the same property also means that organic peroxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds, and this process has been used in explosives. Organic peroxides, like their inorganic counterparts, are powerful bleaching agents.
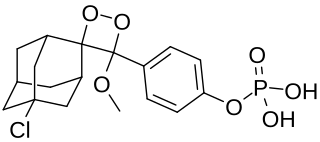
CSPD ([3-(1-chloro-3'-methoxyspiro[adamantane-4,4'-dioxetane]-3'-yl)phenyl] dihydrogen phosphate) is a chemical substance with formula C18H22ClO7P. It is a component of enhanced chemiluminescence enzyme-linked immunosorbent assay (ELISA) kits, used for the detection of minute amounts of various substances such as proteins.
The chemical substance 1,2-dioxetane (1,2-dioxacyclobutane) is a heterocyclic organic compound with formula C2O2H4, containing a ring of two adjacent oxygen atoms and two adjacent carbon atoms. It is therefore an organic peroxide, and can be viewed as a dimer of formaldehyde (COH2).
The molecular formula C3H6O2 may refer to:

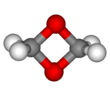
In chemistry, dioxirane is a compound with formula CH
2O
2, whose molecule consists of a ring with one carbon and two oxygen atoms, and two hydrogen atoms attached to the carbon. It is a heterocyclic compound, the smallest cyclic organic peroxide.

The chemical compound 1,2-dioxetanedione, or 1,2-dioxacyclobutane-3,4-dione, often called peroxyacid ester, is an unstable oxide of carbon (an oxocarbon) with formula C2O4. It can be viewed as a double ketone of 1,2-dioxetane (1,2-dioxacyclobutane), or a cyclic dimer of carbon dioxide.
A dioxetane or dioxacyclobutane is an organic compound with formula C2O2H4, whose backbone is a four-membered ring of two oxygen atoms and two carbon atoms. There are two isomers:
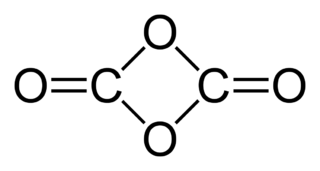
The chemical compound 1,3-dioxetanedione, or 1,3-dioxacyclobutane-2,4-dione is a hypothetical oxide of carbon with formula C2O4. It can be considered a cyclic dimer of carbon dioxide (CO2) or as a double ketone of 1,3-dioxetane (1,3-dioxacyclobutane).
The molecular formula C2O4 may refer to:
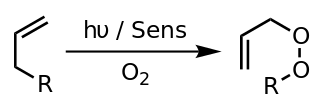
A photooxygenation is a light-induced oxidation reaction in which molecular oxygen is incorporated into the product(s). Initial research interest in photooxygenation reactions arose from Oscar Raab's observations in 1900 that the combination of light, oxygen and photosensitizers is highly toxic to cells. Early studies of photooxygenation focused on oxidative damage to DNA and amino acids, but recent research has led to the application of photooxygenation in organic synthesis and photodynamic therapy.
Eulim or ilm is a Chemistry library written in Ruby under the MIT license. Eulim is a Ruby gem for Chemistry, which supports the calculation of molecular mass of compound, balancing chemical equations, efficient handling of states of chemical species and many more things.

Cyclohexanehexathione is a cyclic covalent compound consisting of a six-carbon ring with a sulfur bonded to each. It has been synthesized by neutralization of its monoanion (C6S6–) in a mass spectrometer. This compound is the thioketone analog of cyclohexanehexone; that oxygen variant is expected to be substantially less stable. Synthesis of C6S6 by photolysis or pyrolysis to extrude three equivalents of carbon monoxide from a precursor containing adjacent pairs of sulfurs as cyclic dithiocarbonate units gave what is more likely a different valence isomer, as various dithiete-containing structures are predicted to be more stable than the hexathione form.
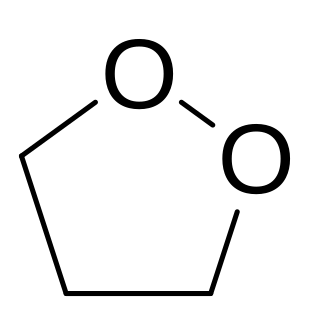
1,2-Dioxolane is a chemical compound with formula C3H6O2, consisting of a ring of three carbon atoms and two oxygen atoms in adjacent positions. Its structural formula could be written as [–(CH
2)3–O–O–].