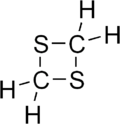 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name 1,3-Dithietane | |
| Systematic IUPAC name 1,3-Dithiacyclobutane | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C2H4S2 | |
| Molar mass | 92.18 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
1,3-Dithietane is a dithietane. It is a colorless, crystalline, unpleasant-smelling solid. It was first prepared in 1976 by the reaction of bis(chloromethyl) sulfoxide with sodium sulfide to give 1,3-dithietane 1-oxide, followed by THF-borane reduction. [1] [2]

Examples of compounds bearing this functional group include the antibiotic Cefotetan and the pesticide Fosthietan. Desaurins are a type of 1,3-dithietanes with alkylidene groups attached to the two carbon atoms.