An acid is a molecule or ion capable of donating a proton (hydrogen ion H+), or, alternatively, capable of forming a covalent bond with an electron pair (a Lewis acid).

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a carbon. The term alcohol originally referred to the primary alcohol ethanol, which is used as a drug and is the main alcohol present in alcoholic beverages. An important class of alcohols, of which methanol and ethanol are the simplest members, includes all compounds for which the general formula is CnH2n+1OH. It is these simple monoalcohols that are the subject of this article.
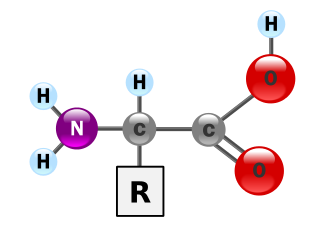
Amino acids are organic compounds that contain amine (-NH2) and carboxyl (-COOH) functional groups, along with a side chain (R group) specific to each amino acid. The key elements of an amino acid are carbon (C), hydrogen (H), oxygen (O), and nitrogen (N), although other elements are found in the side chains of certain amino acids. About 500 naturally occurring amino acids are known (though only 20 appear in the genetic code) and can be classified in many ways. They can be classified according to the core structural functional groups' locations as alpha- (α-), beta- (β-), gamma- (γ-) or delta- (δ-) amino acids; other categories relate to polarity, pH level, and side chain group type (aliphatic, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid residues form the second-largest component (water is the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis.
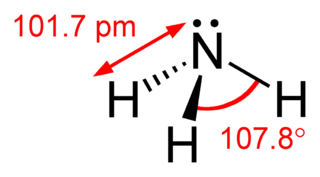
In organic chemistry, amines (, UK also ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; see Category:Amines for a list of amines. Inorganic derivatives of ammonia are also called amines, such as chloramine (NClH2); see Category:Inorganic amines.

Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. Biochemical processes give rise to the complexity of life.
A bactericide or bacteriocide, sometimes abbreviated Bcidal, is a substance that kills bacteria. Bactericides are disinfectants, antiseptics, or antibiotics.

Proteins are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.

Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences.

In chemistry, a zwitterion, formerly called a dipolar ion, is a molecule with two or more functional groups, of which at least one has a positive and one has a negative electrical charge and the net charge of the entire molecule is zero. Because they contain at least one positive and one negative charge, zwitterions are also sometimes called inner salts. The charges on the different functional groups balance each other out, and the molecule as a whole is electrically neutral. The pH where this happens is known as the isoelectric point.

Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Similar to all other amino acids it contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH+
3 form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged aspartate form, −COO−. It is a non-essential amino acid in humans, meaning the body can synthesize it as needed. It is encoded by all the codons GAU and GAC.

Histidine (symbol His or H) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential only for infants, longer-term studies have shown it is essential for adults also. It is encoded by the codons CAU and CAC.
A chaotropic agent is a molecule in water solution that can disrupt the hydrogen bonding network between water molecules. This has an effect on the stability of the native state of other molecules in the solution, mainly macromolecules by weakening the hydrophobic effect. For example, a chaotropic agent reduces the amount of order in the structure of a protein formed by water molecules, both in the bulk and the hydration shells around hydrophobic amino acids, and may cause its denaturation.

1-Propanol is a primary alcohol with the formula CH
3CH
2CH
2OH. This colorless liquid is also known as propan-1-ol, 1-propyl alcohol, n-propyl alcohol, and n-propanol. It is an isomer of 2-propanol. It is formed naturally in small amounts during many fermentation processes and used as a solvent in the pharmaceutical industry, mainly for resins and cellulose esters.

In enzymology, a (R,R)-butanediol dehydrogenase (EC 1.1.1.4) is an enzyme that catalyzes the chemical reaction
Isopropyl alcohol (IUPAC name propan-2-ol; commonly called isopropanol or 2-propanol) is a colorless, flammable chemical compound (chemical formula CH3CHOHCH3) with a strong odor. As an isopropyl group linked to a hydroxyl group, it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of 1-propanol and ethyl methyl ether.

Xanthinol is a drug prepared from theophylline used as a vasodilator. It is most often used as the salt with niacin, known as xantinol nicotinate.
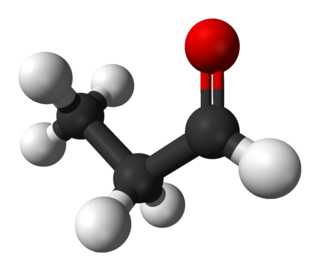
Propionaldehyde or propanal is the organic compound with the formula CH3CH2CHO. It is a saturated 3-carbon aldehyde and is a structural isomer of acetone. It is a colorless liquid with a slightly irritating, fruity odor.

Triisopropanolamine is an amine used for a variety of industrial applications including as an emulsifier, stabilizer, and chemical intermediate. It is also used to neutralize acidic components of some herbicides.

Aminomethyl propanol is an organic compound with the formula H2NC(CH3)2CH2OH. It is colorless liquid that is classified as an alkanolamine. It is a useful buffer and a precursor to numerous other organic compounds.