
The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act, and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."
A wine fault or defect is an unpleasant characteristic of a wine often resulting from poor winemaking practices or storage conditions, and leading to wine spoilage. Many of the compounds that cause wine faults are already naturally present in wine but at insufficient concentrations to be of issue. In fact, depending on perception, these concentrations may impart positive characters to the wine. However, when the concentration of these compounds greatly exceeds the sensory threshold, they replace or obscure the flavors and aromas that the wine should be expressing. Ultimately the quality of the wine is reduced, making it less appealing and sometimes undrinkable.

Toilet Duck is a brand name toilet cleaner noted for the duck-shape of its bottle, so shaped to assist in dispensing the cleaner under the rim. The design was patented in the 1980s by Walter Düring from Dällikon, Switzerland. It is now produced by S. C. Johnson & Son.

1-Octen-3-ol, octenol for short and also known as mushroom alcohol, is a chemical that attracts biting insects such as mosquitoes. It is contained in human breath and sweat, and it was once believed that insect repellent DEET worked by blocking the insects' octenol odorant receptors. Recent evidence in Anopheles gambiae and Culex quequinfasciatius mosquitoes suggest DEET reduces the volatility of 1-octen-3-ol which can result in a reduction in human attraction. 1-Octen-3-ol is a secondary alcohol derived from 1-octene. It exists in the form of two enantiomers, (R)-(–)-1-octen-3-ol and (S)-(+)-1-octen-3-ol.
In enzymology, a taxane 10beta-hydroxylase (EC 1.14.13.76) is an enzyme that catalyzes the chemical reaction
In enzymology, an indole-3-acetaldehyde oxidase (EC 1.2.3.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-hydroxy-3-isohexenylglutaryl-CoA lyase is an enzyme that catalyzes the chemical reaction
In enzymology, an indoleacetate-lysine synthetase (EC 6.3.2.20) is an enzyme that catalyzes the chemical reaction
In enzymology, a beta-pyrazolylalanine synthase (EC 2.5.1.51) is an enzyme that catalyzes the chemical reaction
In enzymology, a L-mimosine synthase (EC 2.5.1.52) is an enzyme that catalyzes the chemical reaction
In enzymology, an uracilylalanine synthase (EC 2.5.1.53) is an enzyme that catalyzes the chemical reaction
In enzymology, an indole-3-acetate beta-glucosyltransferase is an enzyme that catalyzes the chemical reaction
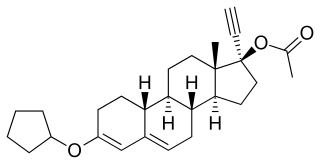
Quingestanol acetate, sold under the brand names Demovis and Pilomin among others, is a progestin medication which was used in birth control pills but is no longer marketed. It is taken by mouth.
Taxoid 14beta-hydroxylase (EC 1.14.13.146) is an enzyme with systematic name 10beta-hydroxytaxa-4(20),11-dien-5alpha-yl-acetate,NADPH:oxygen 14-oxidoreductase. This enzyme catalyses the following chemical reaction
The molecular formula C10H18O2 may refer to:
(Z)-3-hexen-1-ol acetyltransferase is an enzyme with systematic name acetyl-CoA:(3Z)-hex-3-en-1-ol acetyltransferase. This enzyme catalyses the following chemical reaction

ICI-164384, also known as N-n-butyl-N-methyl-11-(3,17β-dihydroxyestra-1,3,5 -trien-7α-yl)undecanamide, is a steroidal antiestrogen and a synthetic derivative of estradiol which is closely related to fulvestrant and was never marketed. It is a silent antagonist of the estrogen receptor (ER) with no intrinsic estrogenic activity and hence is a pure antiestrogen, unlike selective estrogen receptor modulators (SERMs) like tamoxifen. The drug was under development by AstraZeneca for the treatment of breast cancer but was discontinued in favor of fulvestrant, which is very similar to ICI-164384 but is more potent in comparison.

6α-Methylprogesterone (6α-MP) is a progestin which was never marketed. It has 150% of the progestogenic potency of progesterone. In addition, and in contrast to progesterone, 6α-MP has weak androgenic, antiandrogenic, and synandrogenic actions. 6α-MP is structurally related to medroxyprogesterone acetate and megestrol acetate, which possess androgenic and/or antiandrogenic activity to varying degrees similarly. MPA is more androgenic than 6α-MP and MGA.

THC morpholinylbutyrate is a synthetic derivative of tetrahydrocannabinol, developed in the 1970s. It is a prodrug which is converted into THC inside the body, and was one of the first derivatives of THC that is able to form water-soluble salts, giving it a significant advantage over THC for some applications. However, it is less potent than THC and the metabolic conversion to THC is relatively slow and variable, giving it unpredictable pharmacokinetics which has limited its research applications.







