
In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In organic chemistry, a ketene is an organic compound of the form RR'C=C=O, where R and R' are two arbitrary monovalent chemical groups. The name may also refer to the specific compound ethenone H2C=C=O, the simplest ketene.
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure, or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.

Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2(CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to triethylaluminium.

Mevalonic acid (MVA) is a key organic compound in biochemistry; the name is a contraction of dihydroxymethylvalerolactone. The carboxylate anion of mevalonic acid, which is the predominant form in biological environments, is known as mevalonate and is of major pharmaceutical importance. Drugs like statins stop the production of mevalonate by inhibiting HMG-CoA reductase.

ε-Caprolactone or simply caprolactone is a lactone possessing a seven-membered ring. Its name is derived from caproic acid. This colorless liquid is miscible with most organic solvents and water. It was once produced on a large scale as a precursor to caprolactam.
Cycloocta-1,5-diene is a cyclic hydrocarbon with the chemical formula C8H12, specifically [−(CH2)2−CH=CH−]2.
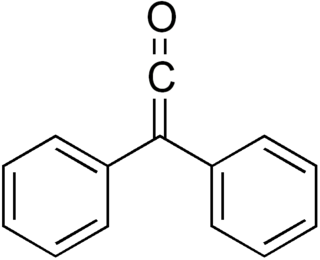
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most stable disubstituted ketenes, is a red-orange oil at room temperature and pressure. Due to the successive double bonds in the ketene structure R1R2C=C=O, diphenyl ketene is a heterocumulene. The most important reaction of diphenyl ketene is the [2+2] cycloaddition at C-C, C-N, C-O, and C-S multiple bonds.

Allylpalladium(II) chloride dimer (APC) is a chemical compound with the formula [(η3-C3H5)PdCl]2. This yellow air-stable compound is an important catalyst used in organic synthesis. It is one of the most widely used transition metal allyl complexes.
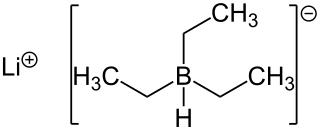
Lithium triethylborohydride is the organoboron compound with the formula LiEt3BH. Commonly referred to as LiTEBH or Superhydride, it is a powerful reducing agent used in organometallic and organic chemistry. It is a colorless or white liquid but is typically marketed and used as a THF solution. The related reducing agent sodium triethylborohydride is commercially available as toluene solutions.
Iodolactonization is an organic reaction that forms a ring by the addition of an oxygen and iodine across a carbon-carbon double bond. It is an intramolecular variant of the halohydrin synthesis reaction. The reaction was first reported by M. J. Bougalt in 1904 and has since become one of the most effective ways to synthesize lactones. Strengths of the reaction include the mild conditions and incorporation of the versatile iodine atom into the product.
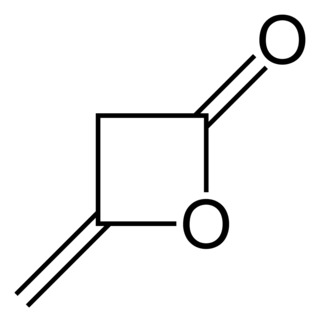
Diketene is an organic compound with the molecular formula C4H4O2, and which is sometimes written as (CH2CO)2. It is formed by dimerization of ketene, H2C=C=O. Diketene is a member of the oxetane family. It is used as a reagent in organic chemistry. It is a colorless liquid.

Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.

Diethylaluminium chloride, abbreviated DEAC, is an organoaluminium compound. Although usually given the chemical formula (C2H5)2AlCl, it exists as a dimer, [(C2H5)2AlCl]2 It is a precursor to Ziegler-Natta catalysts employed for the production of polyolefins. The compound is also a Lewis acid, useful in organic synthesis. The compound is a colorless waxy solid, but is usually handled as a solution in hydrocarbon solvents. It is highly reactive, even pyrophoric.
2,2,4,4-Tetramethyl-3-t-butyl-pentane-3-ol or tri-tert-butylcarbinol is an organic compound with formula C13H28O, ((H3C)3C)3COH, or tBu3COH. It is an alcohol that can be viewed as a structural analog of a tridecane isomer (2,2,4,4-tetramethyl-3-t-butylpentane) where the central hydrogen has been replaced by a hydroxyl group -OH.

Reductions with samarium(II) iodide involve the conversion of various classes of organic compounds into reduced products through the action of samarium(II) iodide, a mild one-electron reducing agent.
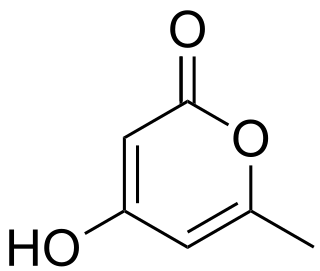
Triacetic acid lactone is an organic compound derived enzymatically from glucose. It is a light yellow solid that is soluble in organic solvents.

Arglabin is a sesquiterpene lactone belonging to the guaianolide subclass bearing a 5,7,5-tricyclic ring system which is known to inhibit farnesyl transferase. It is characterized by an epoxide on the cycloheptane as well as an exocyclic methylene group that is conjugated with the carbonyl of the lactone. Arglabin is extracted from Artemisia glabella, a species of wormwood, found in the Karaganda Region of Kazakhstan. Arglabin and its derivatives are biologically active and demonstrate promising antitumor activity and cytoxocity against varying tumor cell lines.
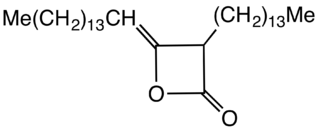
Alkyl ketene dimers (AKDs) are a family of organic compounds based on the 4-membered ring system of oxetan-2-one, which is also the central structural element of propiolactone and diketene. Attached to the oxetane ring of technically relevant alkyl ketene dimers there is a C12 – C16 alkyl group in the 3-position and a C13 – C17 alkylidene group in the 4-position.

Isobutyryl chloride is the organic compound with the formula (CH3)2CHCOCl. A colorless liquid, it the simplest branched-chain acyl chloride. It is prepared by chlorination of isobutyric acid.

















