In polymer chemistry, polymerization, or polymerisation, is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them.
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar. Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups.
In polymer chemistry, ring-opening polymerization (ROP) is a form of chain-growth polymerization, in which the terminus of a polymer chain attacks cyclic monomers to form a longer polymer. The reactive center can be radical, anionic or cationic. Some cyclic monomers such as norbornene or cyclooctadiene can be polymerized to high molecular weight polymers by using metal catalysts. ROP is a versatile method for the synthesis of biopolymers.
Emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomer, and surfactant. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer are emulsified in a continuous phase of water. Water-soluble polymers, such as certain polyvinyl alcohols or hydroxyethyl celluloses, can also be used to act as emulsifiers/stabilizers. The name "emulsion polymerization" is a misnomer that arises from a historical misconception. Rather than occurring in emulsion droplets, polymerization takes place in the latex/colloid particles that form spontaneously in the first few minutes of the process. These latex particles are typically 100 nm in size, and are made of many individual polymer chains. The particles are prevented from coagulating with each other because each particle is surrounded by the surfactant ('soap'); the charge on the surfactant repels other particles electrostatically. When water-soluble polymers are used as stabilizers instead of soap, the repulsion between particles arises because these water-soluble polymers form a 'hairy layer' around a particle that repels other particles, because pushing particles together would involve compressing these chains.
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such as polymer synthesis. Typical examples are molecules with a nitrogen-halogen bond, azo compounds, and organic and inorganic peroxides.
Chain-growth polymerization (AE) or chain-growth polymerisation (BE) is a polymerization technique where unsaturated monomer molecules add onto the active site on a growing polymer chain one at a time. There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics.
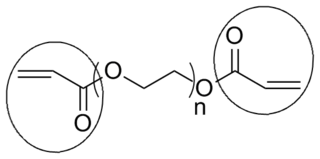
End groups are an important aspect of polymer synthesis and characterization. In polymer chemistry, they are functional groups that are at the very ends of a macromolecule or oligomer (IUPAC). In polymer synthesis, like condensation polymerization and free-radical types of polymerization, end-groups are commonly used and can be analyzed by nuclear magnetic resonance (NMR) to determine the average length of the polymer. Other methods for characterization of polymers where end-groups are used are mass spectrometry and vibrational spectrometry, like infrared and raman spectroscopy. These groups are important for the analysis of polymers and for grafting to and from a polymer chain to create a new copolymer. One example of an end group is in the polymer poly(ethylene glycol) diacrylate where the end-groups are circled.
Free-radical polymerization (FRP) is a method of polymerization, by which a polymer forms by the successive addition of free-radical building blocks. Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain.
Anionic addition polymerization is a form of chain-growth polymerization or addition polymerization that involves the polymerization of monomers initiated with anions. The type of reaction has many manifestations, but traditionally vinyl monomers are used. Often anionic polymerization involves living polymerizations, which allows control of structure and composition.
Atom transfer radical polymerization (ATRP) is an example of a reversible-deactivation radical polymerization. Like its counterpart, ATRA, or atom transfer radical addition, ATRP is a means of forming a carbon-carbon bond with a transition metal catalyst. Polymerization from this method is called atom transfer radical addition polymerization (ATRAP). As the name implies, the atom transfer step is crucial in the reaction responsible for uniform polymer chain growth. ATRP was independently discovered by Mitsuo Sawamoto and by Krzysztof Matyjaszewski and Jin-Shan Wang in 1995.

Reversible addition−fragmentation chain-transfer or RAFT polymerization is one of several kinds of reversible-deactivation radical polymerization. It makes use of a chain-transfer agent in the form of a thiocarbonylthio compound to afford control over the generated molecular weight and polydispersity during a free-radical polymerization. Discovered at the Commonwealth Scientific and Industrial Research Organisation (CSIRO) of Australia in 1998, RAFT polymerization is one of several living or controlled radical polymerization techniques, others being atom transfer radical polymerization (ATRP) and nitroxide-mediated polymerization (NMP), etc. RAFT polymerization uses thiocarbonylthio compounds, such as dithioesters, thiocarbamates, and xanthates, to mediate the polymerization via a reversible chain-transfer process. As with other controlled radical polymerization techniques, RAFT polymerizations can be performed with conditions to favor low dispersity and a pre-chosen molecular weight. RAFT polymerization can be used to design polymers of complex architectures, such as linear block copolymers, comb-like, star, brush polymers, dendrimers and cross-linked networks.
Autoxidation refers to oxidations brought about by reactions with oxygen at normal temperatures, without the intervention of flame or electric spark. The term is usually used to describe the gradual degradation of organic compounds in air at ambient temperatures. Many common phenomena can be attributed to autoxidation, such as food going rancid, the 'drying' of varnishes and paints, and the perishing of rubber. It is also an important concept in both industrial chemistry and biology. Autoxidation is therefore a fairly broad term and can encompass examples of photooxygenation and catalytic oxidation.
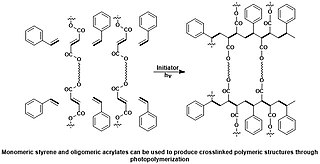
A photopolymer or light-activated resin is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing.
Polymer stabilizers are chemical additives which may be added to polymeric materials, such as plastics and rubbers, to inhibit or retard their degradation. Common polymer degradation processes include oxidation, UV-damage, thermal degradation, ozonolysis, combinations thereof such as photo-oxidation, as well as reactions with catalyst residues, dyes, or impurities. All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-linking, which adversely affects many key properties such as strength, malleability, appearance and colour.

In chemistry, a free radical is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes.
Cobalt based catalysts, when used in radical polymerization, have several main advantages especially in slowing down the reaction rate, allowing for the synthesis of polymers with peculiar properties. As starting the reaction does need a real radical initiator, the cobalt species is not the only used catalyst, it is a mediator. For this reason this type of reaction is referred to as cobalt mediated radical polymerization.
Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term "reversible-deactivation radical polymerization" instead of "living free radical polymerization", though the two terms are not synonymous.
In chemistry, cationic polymerization is a type of chain growth polymerization in which a cationic initiator transfers charge to a monomer which then becomes reactive. This reactive monomer goes on to react similarly with other monomers to form a polymer. The types of monomers necessary for cationic polymerization are limited to alkenes with electron-donating substituents and heterocycles. Similar to anionic polymerization reactions, cationic polymerization reactions are very sensitive to the type of solvent used. Specifically, the ability of a solvent to form free ions will dictate the reactivity of the propagating cationic chain. Cationic polymerization is used in the production of polyisobutylene and poly(N-vinylcarbazole) (PVK).
Reversible deactivation radical polymerizations (RDRPs) are members of the class of reversible deactivation polymerizations which exhibit much of the character of living polymerizations, but cannot be categorized as such as they are not without chain transfer or chain termination reactions. Several different names have been used in literature, which are:
Ionic polymerization is a chain-growth polymerization in which active centers are ions or ion pairs. It can be considered as an alternative to radical polymerization, and may refer to anionic polymerization or cationic polymerization.




