 | |
| Names | |
|---|---|
| IUPAC name Butane-2,3-diamine | |
| Other names 1,2-Dimethylethylenediamine 2,3-Diaminobutane | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID | |
| UNII |
|
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C4H12N2 | |
| Molar mass | 88.154 g·mol−1 |
| Appearance | colorless oil |
| Boiling point | 44-45 °C (25 mmHg, rac) 46-48 °C (25 mmHg, meso) [1] 55.3-59.3 °C (60 mmHg, DL-threo) 56.1-60.5 °C (60 mmHg, meso) [2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
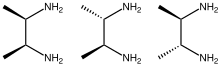
2,3-Butanediamine are organic compounds with the formula CH3CH(NH2)CH(NH2)CH3. Three stereoisomers exist, meso and a pair of enantiomers. These diamines form complexes with transition metals. [3]
![Structure of the cation [Co(meso-bn)2CO3] as determined by X-ray crystallography. Color code: red = N, blue = N. CAWHUD.png](http://upload.wikimedia.org/wikipedia/commons/thumb/6/62/CAWHUD.png/250px-CAWHUD.png)