In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer.

Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.

A meso compound or meso isomer is an optically inactive isomer in a set of stereoisomers, at least two of which are optically active. This means that despite containing two or more stereocenters, the molecule is not chiral. A meso compound is superposable on its mirror image. Two objects can be superposed if all aspects of the objects coincide and it does not produce a "(+)" or "(-)" reading when analyzed with a polarimeter. The name is derived from the Greek mésos meaning “middle”.
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA is also a common abbreviation. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed in organic synthesis, usually as a base.

(E)-Stilbene, commonly known as trans-stilbene, is an organic compound represented by the condensed structural formula C6H5CH=CHC6H5. Classified as a diarylethene, it features a central ethylene moiety with one phenyl group substituent on each end of the carbon–carbon double bond. It has an (E) stereochemistry, meaning that the phenyl groups are located on opposite sides of the double bond, the opposite of its geometric isomer, cis-stilbene. Trans-stilbene occurs as a white crystalline solid at room temperature and is highly soluble in organic solvents. It can be converted to cis-stilbene photochemically, and further reacted to produce phenanthrene.

The Trost ligand is a diphosphine used in the palladium-catalyzed Trost asymmetric allylic alkylation. Other C2-symmetric ligands derived from trans-1,2-diaminocyclohexane (DACH) have been developed, such as the (R,R)-DACH-naphthyl ligand derived from 2-diphenylphosphino-1-naphthalenecarboxylic acid. Related bidentate phosphine-containing ligands derived from other chiral diamines and 2-diphenylphosphinobenzoic acid have also been developed for applications in asymmetric synthesis.

Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.
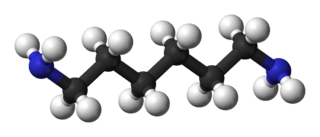
Hexamethylenediamine or hexane-1,6-diamine, is the organic compound with the formula H2N(CH2)6NH2. The molecule is a diamine, consisting of a hexamethylene hydrocarbon chain terminated with amine functional groups. The colorless solid (yellowish for some commercial samples) has a strong amine odor. About 1 billion kilograms are produced annually.
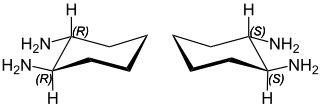
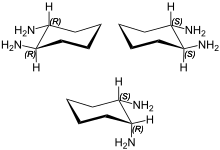
trans-1,2-Diaminocyclohexane is an organic compound with the formula C6H10(NH2)2. This diamine is a building block for C2-symmetric ligands that are useful in asymmetric catalysis.

1,2-Diphenyl-1,2-ethylenediamine, DPEN, is an organic compound with the formula H2NCHPhCHPhNH2, where Ph is phenyl (C6H5). DPEN exists as three stereoisomers: meso and two enantiomers S,S- and R,R-. The chiral diastereomers are used in asymmetric hydrogenation. Both diastereomers are bidentate ligands.

Jacobsen's catalyst is the common name for N,N'-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminomanganese(III) chloride, a coordination compound of manganese and a salen-type ligand. It is used as an asymmetric catalyst in the Jacobsen epoxidation, which is renowned for its ability to enantioselectively transform prochiral alkenes into epoxides. Before its development, catalysts for the asymmetric epoxidation of alkenes required the substrate to have a directing functional group, such as an alcohol as seen in the Sharpless epoxidation. This compound has two enantiomers, which give the appropriate epoxide product from the alkene starting material.

Bisphenol A diglycidyl ether is an organic compound and is a liquid epoxy resin. The compound is a colorless viscous liquid. It is a key component of many epoxy resin formulations. Addition of further Bisphenol A and a catalyst and heat can produce Bisphenol A glycidyl ether epoxy resins of higher molecular weight that are solid.

1,2-Diaminopropane (propane-1,2-diamine) is organic compound with the formula CH3CH(NH2)CH2NH2. A colorless liquid, it is the simplest chiral diamine. It is used as a bidentate ligand in coordination chemistry.
4,4'-Diaminodicyclohexylmethane is the name for organic compounds with the formula CH2(C6H10NH2)2. It is classified as a diamine. In the epoxy industry it is often referred to as PACM, short for para-diaminodicyclohexylmethane. It is used as a curing agent for epoxy resins It finds particular use in epoxy flooring. Another use is to produce diisocyanates, which are precursors to polyurethanes. The mixture is a colorless solid, but typical samples are yellowish and oily. The compound is produced as a mixture of three isomers by the hydrogenation of methylenedianiline. These isomers are, in decreasing order of their yield from the hydrogenation, trans-trans, cis-trans, and a small amount of cis-cis.

Isophorone diamine (usually shortened to IPDA) is a chemical compound and specifically a diamine with the formula (CH3)3C6H7(NH2)(CH2NH2). It is a colorless liquid. It is a precursor to polymers and coatings.

1,3-bis(aminomethyl)cyclohexane (1,3-BAC) are a collection of organic compounds with the formula C6H10(CH2NH2)2. The compounds belong to the sub class cycloaliphatic amine. Their key use is as an epoxy resin curing agent.

2-Methylpentamethylenediamine is an organic compound part of the amine family with the formula H2NCH2CH2CH2CH(CH3)CCH2NCH2. A colorless liquid, this diamine is obtained by the hydrogenation of 2-methylglutaronitrile. It is better known by the trade name "Dytek A".
In organic chemistry, amine value is a measure of the nitrogen content of an organic molecule. Specifically, it is usually used to measure the amine content of amine functional compounds. It may be defined as the number of milligrams of potassium hydroxide (KOH) equivalent to one gram of epoxy hardener resin. The units are thus mg KOH/g.
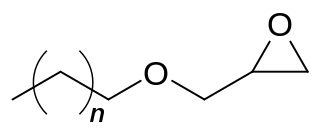
C12-C13 alcohol glycidyl ether is a mixture of organic chemicals in the glycidyl ether family. It is a mixture of mainly 12 and 13 carbon chain alcohols, also called fatty alcohols that have been glycidated. It is an industrial chemical used as a surfactant but primarily for epoxy resin viscosity reduction. It has the CAS number 120547-52-6.
2,3-Butanediamine are organic compounds with the formula CH3CH(NH2)CH(NH2)CH3. Three stereoisomers exist, meso and a pair of enantiomers. These diamines form complexes with transition metals.