A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes which alter membrane fluidity; and as signaling molecules. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols lanosterol (opisthokonts) or cycloartenol (plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene.
Hydroxylation is a chemical process that introduces a hydroxyl group (-OH) into an organic compound. In biochemistry, hydroxylation reactions are often facilitated by enzymes called hydroxylases. Hydroxylation is the first step in the oxidative degradation of organic compounds in air. It is extremely important in detoxification since hydroxylation converts lipophilic compounds into water-soluble (hydrophilic) products that are more readily removed by the kidneys or liver and excreted. Some drugs are activated or deactivated by hydroxylation.

Gingerol, properly as [6]-gingerol, is a phenol phytochemical compound found in fresh ginger that activates spice receptors on the tongue. Molecularly, gingerol is a relative of capsaicin and piperine, the compounds which are alkaloids, though the bioactive pathways are unconnected. It is normally found as a pungent yellow oil in the ginger rhizome, but can also form a low-melting crystalline solid. This chemical compound is found in all members of the Zingiberaceae family plant and is high in concentrations in the grains of paradise as well as an African Ginger species.

Bile acids are steroid acids found predominantly in the bile of mammals and other vertebrates. Diverse bile acids are synthesized in the liver. Bile acids are conjugated with taurine or glycine residues to give anions called bile salts.

Mitotane, sold under the brand name Lysodren, is a steroidogenesis inhibitor and cytostatic antineoplastic medication which is used in the treatment of adrenocortical carcinoma and Cushing's syndrome. It is a derivative of the early insecticide DDT and an isomer of p,p'-DDD (4,4'-dichlorodiphenyldichloroethane) and is also known as 2,4'-(dichlorodiphenyl)-2,2-dichloroethane (o,p'-DDD).

Tyrosine hydroxylase or tyrosine 3-monooxygenase is the enzyme responsible for catalyzing the conversion of the amino acid L-tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA). It does so using molecular oxygen (O2), as well as iron (Fe2+) and tetrahydrobiopterin as cofactors. L-DOPA is a precursor for dopamine, which, in turn, is a precursor for the important neurotransmitters norepinephrine (noradrenaline) and epinephrine (adrenaline). Tyrosine hydroxylase catalyzes the rate limiting step in this synthesis of catecholamines. In humans, tyrosine hydroxylase is encoded by the TH gene, and the enzyme is present in the central nervous system (CNS), peripheral sympathetic neurons and the adrenal medulla. Tyrosine hydroxylase, phenylalanine hydroxylase and tryptophan hydroxylase together make up the family of aromatic amino acid hydroxylases (AAAHs).

CYP27A1 is a gene encoding a cytochrome P450 oxidase, and is commonly known as sterol 27-hydroxylase. This enzyme is located in many different tissues where it is found within the mitochondria. It is most prominently involved in the biosynthesis of bile acids.

Cholesterol 7 alpha-hydroxylase also known as cholesterol 7-alpha-monooxygenase or cytochrome P450 7A1 (CYP7A1) is an enzyme that in humans is encoded by the CYP7A1 gene which has an important role in cholesterol metabolism. It is a cytochrome P450 enzyme, which belongs to the oxidoreductase class, and converts cholesterol to 7-alpha-hydroxycholesterol, the first and rate limiting step in bile acid synthesis.

Tryptophan hydroxylase (TPH) is an enzyme (EC 1.14.16.4) involved in the synthesis of the neurotransmitter serotonin. Tyrosine hydroxylase, phenylalanine hydroxylase, and tryptophan hydroxylase together constitute the family of biopterin-dependent aromatic amino acid hydroxylases. TPH catalyzes the following chemical reaction

The liver receptor homolog-1 (LRH-1) also known as NR5A2 is a protein that in humans is encoded by the NR5A2 gene. LRH-1 is a member of the nuclear receptor family of intracellular transcription factors.

Canrenone, sold under the brand names Contaren, Luvion, Phanurane, and Spiroletan, is a steroidal antimineralocorticoid of the spirolactone group related to spironolactone which is used as a diuretic in Europe, including in Italy and Belgium. It is also an important active metabolite of spironolactone, and partially accounts for its therapeutic effects.

Cholesterol 24-hydroxylase, also commonly known as cholesterol 24S-hydroxylase, cholesterol 24-monooxygenase, CYP46, or CYP46A1, is an enzyme that catalyzes the conversion of cholesterol to 24S-hydroxycholesterol. It is responsible for the majority of cholesterol turnover in the human central nervous system. The systematic name of this enzyme class is cholesterol,NADPH:oxygen oxidoreductase (24-hydroxylating).
In enzymology, a cholesterol 7alpha-monooxygenase (EC 1.14.13.17) is an enzyme that catalyzes the chemical reaction

25-hydroxycholesterol 7-alpha-hydroxylase also known as oxysterol and steroid 7-alpha-hydroxylase is an enzyme that in humans is encoded by the CYP7B1 gene. This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids.

CYP8B1 also known as sterol 12-alpha-hydroxylase is a protein which in humans is encoded by the CYP8B1 gene.

7α-Hydroxy-4-cholesten-3-one is an intermediate in the biochemical synthesis of bile acids from cholesterol. Its precursor, 7α-hydroxycholesterol, is produced from cholesterol by hepatic cholesterol 7α-hydroxylase (CYP7A1).
25-hydroxycholesterol 7alpha-hydroxylase (EC 1.14.13.100, 25-hydroxycholesterol 7alpha-monooxygenase, CYP7B1, CYP7B1 oxysterol 7alpha-hydroxylase) is an enzyme with systematic name cholest-5-ene-3beta,25-diol,NADPH:oxygen oxidoreductase (7alpha-hydroxylating). This enzyme catalyses the following chemical reaction
3-ketosteroid 9alpha-monooxygenase (EC 1.14.13.142, KshAB, 3-ketosteroid 9alpha-hydroxylase) is an enzyme with systematic name androsta-1,4-diene-3,17-dione,NADH:oxygen oxidoreductase (9alpha-hydroxylating). This enzyme catalyses the following chemical reaction
A steroidogenesis inhibitor, also known as a steroid biosynthesis inhibitor, is a type of drug which inhibits one or more of the enzymes that are involved in the process of steroidogenesis, the biosynthesis of endogenous steroids and steroid hormones. They may inhibit the production of cholesterol and other sterols, sex steroids such as androgens, estrogens, and progestogens, corticosteroids such as glucocorticoids and mineralocorticoids, and neurosteroids. They are used in the treatment of a variety of medical conditions that depend on endogenous steroids.
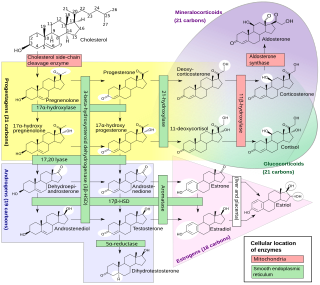
Steroidogenic enzymes are enzymes that are involved in steroidogenesis and steroid biosynthesis. They are responsible for the biosynthesis of the steroid hormones, including sex steroids and corticosteroids, as well as neurosteroids, from cholesterol. Steroidogenic enzymes are most highly expressed in classical steroidogenic tissues, such as the testis, ovary, and adrenal cortex, but are also present in other tissues in the body.