In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)–. The simplest ketone is acetone, with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids (DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres.
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases.

Hydrazine is an inorganic compound with the chemical formula N2H4. It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. It is not dissimilar to Diazene or Triazene and, although its name might suggest otherwise, very dissimilar to Triazine and Tetrazine.
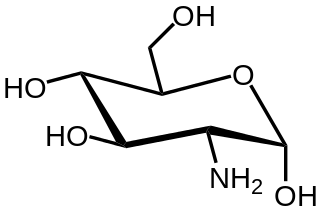
In organic chemistry, an amino sugar is a sugar molecule in which a hydroxyl group has been replaced with an amine group. More than 60 amino sugars are known, with one of the most abundant being N-Acetyl-d-glucosamine, which is the main component of chitin.

In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups. The nitro group is one of the most common explosophores used globally. The nitro group is also strongly electron-withdrawing. Because of this property, C−H bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid.

Hydrazoic acid, also known as hydrogen azide or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.

Organoborane or organoboron compounds are chemical compounds of boron and carbon that are organic derivatives of BH3, for example trialkyl boranes. Organoboron chemistry or organoborane chemistry is the chemistry of these compounds.
The Staudinger reaction is a chemical reaction of an organic azide with a phosphine or phosphite produces an iminophosphorane. The reaction was discovered by and named after Hermann Staudinger. The reaction follows this stoichiometry:

The Einhorn–Brunner reaction is the designation for the chemical reaction of imides with alkyl hydrazines to form an isomeric mixture of 1,2,4-triazoles. It was initially described by the German chemist Alfred Einhorn in a paper, published in 1905, describing N-methylol compounds of amides. In 1914 chemist Karl Brunner published a paper expanding on Einhorn's research of the reaction pictured below, thus resulting in the naming as the Einhorn-Brunner. Substituted 1,2,4-triazole have been prepared from diverse imides and hydrazines.

1,3,5-Triazido-2,4,6-trinitrobenzene, also known as TATNB (triazidotrinitrobenzene) and TNTAZB (trinitrotriazidobenzene), is an aromatic high explosive composed of a benzene ring with three azido groups (-N3) and three nitro groups (-NO2) alternating around the ring, giving the chemical formula C6(N3)3(NO2)3. Its detonation velocity is 7,350 meters per second, which is comparable to TATB (triaminotrinitrobenzene).
Hydrazines (R2N−NR2) are a class of chemical compounds with two nitrogen atoms linked via a covalent bond and which carry from one up to four alkyl or aryl substituents. Hydrazines can be considered as derivatives of the inorganic hydrazine (H2N−NH2), in which one or more hydrogen atoms have been replaced by hydrocarbon groups.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.
The Stieglitz rearrangement is a rearrangement reaction in organic chemistry which is named after the American chemist Julius Stieglitz (1867–1937) and was first investigated by him and Paul Nicholas Leech in 1913. It describes the 1,2-rearrangement of trityl amine derivatives to triaryl imines. It is comparable to a Beckmann rearrangement which also involves a substitution at a nitrogen atom through a carbon to nitrogen shift. As an example, triaryl hydroxylamines can undergo a Stieglitz rearrangement by dehydration and the shift of a phenyl group after activation with phosphorus pentachloride to yield the respective triaryl imine, a Schiff base.
Electrophilic amination is a chemical process involving the formation of a carbon–nitrogen bond through the reaction of a nucleophilic carbanion with an electrophilic source of nitrogen.
In organic chemistry, thiocarboxylic acids are organosulfur compounds related to carboxylic acids by replacement of one of the oxygen atoms with a sulfur atom. Two tautomers are possible: a thione form and a thiol form. These are sometimes also referred to as "carbothioic O-acid" and "carbothioic S-acid" respectively. Of these the thiol form is most common.

4-Chlorophenyl azide is an organic aryl azide compound with the chemical formula C6H4ClN3. The geometry between the nitrogen atoms in the azide functional group is approximately linear while the geometry between the nitrogen and the carbon of the benzene is trigonal planar.
The Blum–Ittah aziridine synthesis, also known as the Blum–Ittah-Shahak aziridine synthesis or simply the Blum aziridine synthesis is a name reaction of organic chemistry, for the generation of aziridines from oxiranes.
An organic azide is an organic compound that contains an azide functional group. Because of the hazards associated with their use, few azides are used commercially although they exhibit interesting reactivity for researchers. Low molecular weight azides are considered especially hazardous and are avoided. In the research laboratory, azides are precursors to amines. They are also popular for their participation in the "click reaction" and in Staudinger ligation. These two reactions are generally quite reliable, lending themselves to combinatorial chemistry.