 | |
 | |
| Names | |
|---|---|
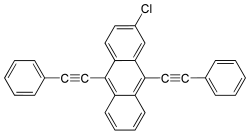
| Preferred IUPAC name 2-Chloro-9,10-bis(phenylethynyl)anthracene | |
| Other names 2-Chloro-BPEA | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.152.467 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C30H17Cl | |
| Molar mass | 412.91 g/mol |
| Melting point | 224 to 226 °C (435 to 439 °F; 497 to 499 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
2-Chloro-9,10-bis(phenylethynyl)anthracene is a fluorescent dye used in lightsticks. It emits green light, used in 12-hour low-intensity Cyalume sticks.