 | |
| Names | |
|---|---|
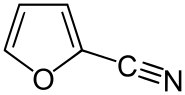
| Preferred IUPAC name Furan-2-carbonitrile | |
| Other names 2-Cyanofuran; 2-Furancarbonitrile; 2-Furyl cyanide | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.009.581 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C5H3NO | |
| Molar mass | 93.085 g·mol−1 |
| Appearance | colorless (yellow if impure) |
| Density | 1.0650 @20 °C [1] |
| Boiling point | 147 [2] °C (297 °F; 420 K) |
| Hazards | |
| Flash point | 35 °C; 95 °F; 308 K |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
2-Furonitrile is a colorless derivative of furan possessing a nitrile group.