
In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres.

Vanillin is an organic compound with the molecular formula C8H8O3. It is a phenolic aldehyde. Its functional groups include aldehyde, hydroxyl, and ether. It is the primary component of the extract of the vanilla bean. Synthetic vanillin is now used more often than natural vanilla extract as a flavoring in foods, beverages, and pharmaceuticals.

An oxime is a chemical compound belonging to the imines, with the general formula RR'C=NOH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.
The Kolbe–Schmitt reaction or Kolbe process is a carboxylation chemical reaction that proceeds by heating sodium phenoxide with carbon dioxide under pressure, then treating the product with sulfuric acid. The final product is an aromatic hydroxy acid which is also known as salicylic acid.

Piperonal, also known as heliotropin, is an organic compound which is commonly found in fragrances and flavors. The molecule is structurally related to other aromatic aldehydes such as benzaldehyde and vanillin.
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly inserts into other bonds.

The Reimer–Tiemann reaction is a chemical reaction used for the ortho-formylation of phenols; with the simplest example being the conversion of phenol to salicylaldehyde. The reaction was discovered by Karl Reimer and Ferdinand Tiemann. The Reimer in question was Karl Reimer (1845-1883) not the lesser known Carl Ludwig Reimer (1856-1921).
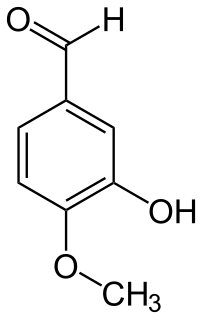
Isovanillin is a phenolic aldehyde, an organic compound and isomer of vanillin. It is a selective inhibitor of aldehyde oxidase. It is not a substrate of that enzyme, and is metabolized by aldehyde dehydrogenase into isovanillic acid, which could make it a candidate drug for use in alcohol aversion therapy. Isovanillin can be used as a precursor in the chemical total synthesis of morphine. The proposed metabolism of isovanillin in rat has been described in literature, and is part of the WikiPathways machine readable pathway collection.
Salicylic aldehyde (2-hydroxybenzaldehyde) is the organic compound with the formula C6H4CHO-2-OH. Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almond odor at higher concentration. Salicylaldehyde is a key precursor to a variety chelating agents, some of which are commercially important.

The Dakin oxidation is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde or ketone reacts with hydrogen peroxide in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidized, and the hydrogen peroxide is reduced.
The Gabriel–Colman rearrangement is the chemical reaction of a saccharin or phthalimido ester with a strong base, such as an alkoxide, to form substituted isoquinolines. First described in 1900 by chemists Siegmund Gabriel and James Colman, this rearrangement, a ring expansion, is seen to be general if there is an enolizable hydrogen on the group attached to the nitrogen, since it is necessary for the nitrogen to abstract a hydrogen to form the carbanion that will close the ring. As shown in the case of the general example below, X is either CO or SO2.
4-Aminobiphenyl (4-APB) is an organic compound with the formula C6H5C6H4NH2. It is an amine derivative of biphenyl. It is a colorless solid, although aged samples can appear colored. 4-Aminobiphenyl was commonly used in the past as a rubber antioxidant and an intermediate for dyes. Exposure to this aryl-amine can happen through contact with chemical dyes and from inhalation of cigarette smoke. Researches showed that 4-aminobiphenyl is responsible for bladder cancer in humans and dogs by damaging DNA. Due to its carcinogenic effects, commercial production of 4-aminobiphenyl ceased in the United States in the 1950s.
Mequinol, MeHQ or 4-methoxyphenol, is a phenol used in dermatology and organic chemistry.

Vanillic acid is a dihydroxybenzoic acid derivative used as a flavoring agent. It is an oxidized form of vanillin. It is also an intermediate in the production of vanillin from ferulic acid.

Johann Karl Wilhelm Ferdinand Tiemann was a German chemist and together with Karl Reimer discoverer of the Reimer-Tiemann reaction.

Gustav Ludwig Friedrich Wilhelm Haarmann was a German chemist and together with Karl Reimer and Ferdinand Tiemann as scientific consultant founded the Haarmann & Reimer chemical plant for the production of vanillin.

ortho-Vanillin (2-hydroxy-3-methoxybenzaldehyde) is an organic solid present in the extracts and essential oils of many plants. Its functional groups include aldehyde, ether and phenol. ortho-Vanillin, a compound of the formula C8H8O3, is distinctly different from its more prevalent isomer, meta-vanillin. The "ortho-" prefix refers to the position of the compound’s hydroxyl moiety, which is found in the para-position in vanillin.
In 1976, the Italian chemist, Giovanni Piancatelli and coworkers developed a new method to synthesize 4-hydroxycyclopentenone derivatives from 2-furylcarbinols through an acid-catalyzed rearrangement. This discovery occurred when Piancatelli was studying heterocyclic steroids and their reactive abilities in an acidic environment. As this rearrangement has continued to be studied, it has become a commonly used rearrangement in natural product synthesis because of the ability to create 4-hydroxy-5-substitutedcyclopent-2-enones. Piancatelli’s motive for looking into this new rearrangement stemmed from the ever present 3-oxycyclopentene molecule, specifically its 5-hydroxy derivative, found in biologically active natural products.
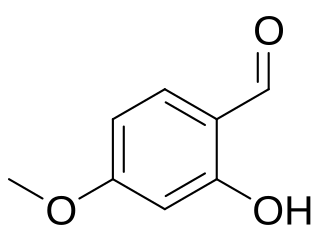
2-Hydroxy-4-methoxybenzaldehyde is a chemical compound and an isomer of vanillin. Urolithin M7, one of the urolithins, has also been synthesized from 2-hydroxy-4-methoxybenzaldehyde using the inverse electron-demand Diels–Alder reaction.
The Fiesselmann thiophene synthesis is a name reaction in organic chemistry that allows for the generation of 3-hydroxy-2-thiophenecarboxylic acid derivatives from α,β-acetylenic esters with thioglycolic acid and its derivatives under the presence of a base. The reaction was developed by Hans Fiesselmann in the 1950s.












