
Butanone, also known as methyl ethyl ketone (MEK) or ethyl methyl ketone, is an organic compound with the formula CH3C(O)CH2CH3. This colorless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran.

Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent.
Methyl ethyl ketone peroxide (MEKP) is an organic peroxide with the formula [(CH3)(C2H5)C(O2H)]2O2. MEKP is a colorless oily liquid. It is widely used in vulcanization (crosslinking) of polymers.
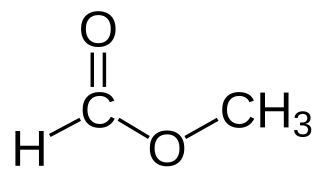
Methyl formate, also called methyl methanoate, is the methyl ester of formic acid. The simplest example of a carboxylate ester, it is a colorless liquid with an ethereal odour, high vapor pressure, and low surface tension. It is a precursor to many other compounds of commercial interest.

3-Pentanone is a simple, symmetrical dialkyl ketone. It is a colorless liquid ketone with an odor like that of acetone. It is soluble in about 25 parts water, but miscible with organic solvents.
Dimethylformamide is an organic compound with the formula (CH3)2N−C(=O)H. Commonly abbreviated as DMF, this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions. Dimethylformamide is odorless, but technical-grade or degraded samples often have a fishy smell due to impurity of dimethylamine. Dimethylamine degradation impurities can be removed by sparging samples with an inert gas such as argon or by sonicating the samples under reduced pressure. As its name indicates, it is structurally related to formamide, having two methyl groups in the place of the two hydrogens. DMF is a polar (hydrophilic) aprotic solvent with a high boiling point. It facilitates reactions that follow polar mechanisms, such as SN2 reactions.

Butan-2-ol, or sec-butanol, is an organic compound with formula CH3CH(OH)CH2CH3. Its structural isomers are 1-butanol, isobutanol, and tert-butanol. 2-Butanol is chiral and thus can be obtained as either of two stereoisomers designated as (R)-(−)-butan-2-ol and (S)-(+)-butan-2-ol. It is normally encountered as a 1:1 mixture of the two stereoisomers — a racemic mixture.
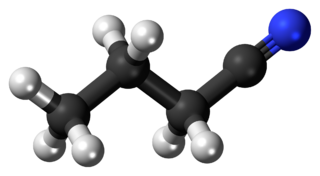
Butyronitrile or butanenitrile or propyl cyanide, is a nitrile with the formula C3H7CN. This colorless liquid is miscible with most polar organic solvents.

Methyl isobutyl ketone (MIBK, 4-methylpentan-2-one) is an organic compound with the condensed chemical formula (CH3)2CHCH2C(O)CH3. This ketone is a colourless liquid that is used as a solvent for gums, resins, paints, varnishes, lacquers, and nitrocellulose.

Mesityl oxide is a α,β-unsaturated ketone with the formula CH3C(O)CH=C(CH3)2. This compound is a colorless, volatile liquid with a honey-like odor.

Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.

n-Butyl acetate is an organic compound with the formula CH3CO2(CH2)3CH3. A colorless, flammable liquid, it is the ester derived from n-butanol and acetic acid. It is found in many types of fruit, where it imparts characteristic flavors and has a sweet smell of banana or apple. It is used as an industrial solvent.

Crotonaldehyde is a chemical compound with the formula CH3CH=CHCHO. The compound is usually sold as a mixture of the E- and Z-isomers, which differ with respect to the relative position of the methyl and formyl groups. The E-isomer is more common (data given in Table is for the E-isomer). This lachrymatory liquid is moderately soluble in water and miscible in organic solvents. As an unsaturated aldehyde, crotonaldehyde is a versatile intermediate in organic synthesis. It occurs in a variety of foodstuffs, e.g. soybean oils.

Isophorone is an α,β-unsaturated cyclic ketone. It is a colorless liquid with a characteristic peppermint-like odor, although commercial samples can appear yellowish. Used as a solvent and as a precursor to polymers, it is produced on a large scale industrially.
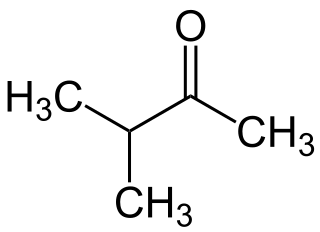
3-Methyl-2-butanone is a ketone and solvent of minor importance. It is comparable to MEK, but has a lower solvency and is more expensive.
Methylcyclohexane (cyclohexylmethane) is an organic compound with the molecular formula is CH3C6H11. Classified as saturated hydrocarbon, it is a colourless liquid with a faint odor. Methylcyclohexane is used as a solvent. It is mainly converted in naphtha reformers to toluene. Methylcyclohexane is also used in some correction fluids (such as White-Out) as a solvent.
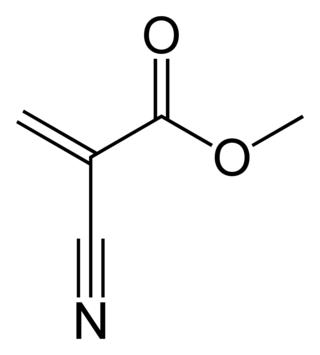
Methyl cyanoacrylate is an organic compound that contains several functional groups: a methyl ester, a nitrile, and an alkene. It is a colorless liquid with low viscosity. Its chief use is as the main component of cyanoacrylate glues. It can be encountered under many trade names. Methyl cyanoacrylate is less commonly encountered than ethyl cyanoacrylate.

2-Hexanone is a ketone used as a general solvent and in paints. It dissolves cellulose nitrate, vinyl polymers and copolymers, and natural and synthetic resins. It is recommended as a solvent because it is photochemically inactive; however it has a very low safe threshold limit value. 2-Hexanone is absorbed through the lungs, orally and dermally and its metabolite, 2,5-hexanedione, is neurotoxic. Animal tests have shown that the neurotoxic effect of 2-hexanone may be potentiated by simultaneous administration of 2-butanone.
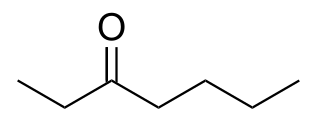
3-Heptanone, is a seven carbon ketone. It is a colorless liquid with a "green odor," also described to have a fruity scent. It is often used as a perfume/fragrance, as a solvent for cellulose, nitrocellulose, or vinyl resins, and as a synthetic building block in the preparation of other organic molecules.

3-Methyl-2-pentanone is an aliphatic ketone and isomer of 2-hexanone. It is used as a solvent and as an intermediate for syntheses. Its industrial importance is low. It is produced by base-catalyzed aldol condensation of 2-butanone with acetaldehyde, forming 4-hydroxy-3-methyl-2-pentanone, which is dehydrated to 3-methyl-3-penten-2-one over an acid catalyst, followed by hydrogenation over a palladium catalyst.


















