
Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.

In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure or mixing with a catalyst. Heat is not necessarily applied externally, and is often generated by the reaction of the resin with a curing agent. Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network.
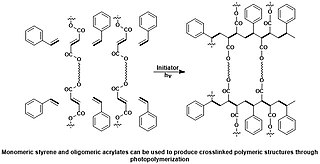
A photopolymer or light-activated resin is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing.
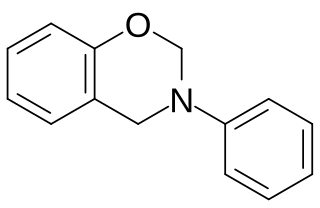
Polybenzoxazines, also called benzoxazine resins, are cured polymerization products derived from benzoxazine monomers.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle.
In polymer chemistry, the term prepolymer or pre-polymer, refers to a monomer or system of monomers that have been reacted to an intermediate-molecular mass state. This material is capable of further polymerization by reactive groups to a fully cured, high-molecular-mass state. As such, mixtures of reactive polymers with un-reacted monomers may also be referred to as pre-polymers. The term "pre-polymer" and "polymer precursor" may be interchanged.
Expanding monomers are monomers which increase in volume (expand) during polymerization. They can be added to monomer formulations to counteract the usual volume shrinking to manufacture products with higher quality and durability. Volume Shrinkage is in first line for the unmeltable thermosets a problem, since those are of fixed shape after polymerization completed.

3,4-Epoxycyclohexanecarboxylate methyl ester refers to organic compounds with the formula C6H9OCO2CH3. These are bifunctional compounds consisting of an ester and cycloaliphatic epoxide groups. It exists as two diastereomers both of which are chiral. These species are of interest in polymer chemistry.
In materials science, a polymer matrix composite (PMC) is a composite material composed of a variety of short or continuous fibers bound together by a matrix of organic polymers. PMCs are designed to transfer loads between fibers of a matrix. Some of the advantages with PMCs include their light weight, high resistance to abrasion and corrosion, and high stiffness and strength along the direction of their reinforcements.
Polyurethane dispersion, or PUD, is understood to be a polyurethane polymer resin dispersed in water, rather than a solvent, although some cosolvent maybe used. Its manufacture involves the synthesis of polyurethanes having carboxylic acid functionality or nonionic hydrophiles like PEG incorporated into, or pendant from, the polymer backbone. Two component polyurethane dispersions are also available.
Waterborne resins are sometimes called water-based resins. They are resins or polymeric resins that use water as the carrying medium as opposed to solvent or solvent-less. Resins are used in the production of coatings, adhesives, sealants, elastomers and composite materials. When the phrase waterborne resin is used, it usually describes all resins which have water as the main carrying solvent. The resin could be water-soluble, water reducible or water dispersed.
Neopentyl glycol diglycidyl ether (NPGDGE) is an organic chemical in the glycidyl ether family. It is aliphatic and a colorless liquid. It has the formula C11H20O4 and the CAS registry number of 17557-23-2. It has two oxirane groups per molecule. Its principle use is in modifying epoxy resins.
1,6-Hexanediol diglycidyl ether is an organic chemical in the glycidyl ether family. It is an aliphatic compound that is a colorless liquid. It has two epoxide (oxirane) groups per molecule. Its main use is in modifying epoxy resins especially viscosity reduction whilst flexibilizing. It is REACH registered.
1,4-Cyclohexanedimethanol diglycidyl ether is an organic chemical in the glycidyl ether family. Its formula is C14H24O4 and the IUPAC name is 2-[[4-(oxiran-2-ylmethoxymethyl)cyclohexyl]methoxymethyl]oxirane. It has the CAS number of 14228-73-0 and is REACH registered in Europe. An industrial chemical, a key use is in the reduction of the viscosity of epoxy resin systems functioning as a reactive diluent.

Trimethylolpropane triglycidyl ether (TMPTGE) is an organic chemical in the glycidyl ether family. It has the formula C15H26O6 and the IUPAC name is 2-[2,2-bis(oxiran-2-ylmethoxymethyl)butoxymethyl]oxirane, and the CAS number 3454-29-3. It also has another CAS number of 30499-70-8 A key use is as a modifier for epoxy resins as a reactive diluent.

Castor oil glycidyl ether is a liquid organic chemical in the glycidyl ether family. It is sometimes called castor oil triglycidyl ether. It has the theoretical formula C66H116O12 and the CAS number 14228-73-0. The IUPAC name is 2,3-bis[12-(oxiran-2-ylmethoxy)octadec-9-enoyloxy]propyl 12-(oxiran-2-ylmethoxy)octadec-9-enoate. A key use is acting as a modifier for epoxy resins as a reactive diluent that adds flexibility and improved mechanical properties.

Trimethylolethane triglycidyl ether (TMETGE) is an organic chemical in the glycidyl ether family. It has the formula C14H24O6 and the IUPAC name is 2-({2-methyl-3-[(oxiran-2-yl)methoxy]-2-{[(oxiran-2-yl)methoxy]methyl}propoxy}methyl)oxirane. The CAS number is 68460-21-9. A key use is as a modifier for epoxy resins as a reactive diluent.

Poly(propylene glycol) diglycidyl ether (PPGDGE) is an organic chemical in the glycidyl ether family. There are a number of variations depending on the starting molecular weight of the polypropylene glycol. They have the formula (C3H6O)n.C6H10O3 and the IUPAC name is Poly[oxy(methyl-1,2-ethanediyl)],a-(2-oxiranylmethyl)-w-(2-oxiranylmethoxy)- A key use is as a modifier for epoxy resins as a reactive diluent and flexibilizer. It is REACH registered.
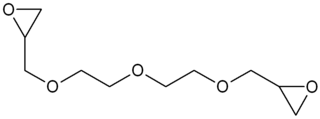
Diethylene glycol diglycidyl ether (DEGDGE) is an organic chemical in the glycidyl ether family with the formula C10H18O5.. The oxirane functionality makes it useful as a reactive diluent for epoxy resin viscosity reduction.

Diglycidyl resorcinol ether, also called Resorcinol diglycidyl ether (RDGE) is a liquid aromatic organic chemical compound and chemically a glycidyl ether.











