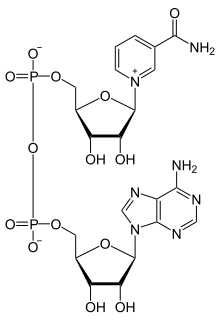
Nicotinamide adenine dinucleotide (NAD) is a cofactor that is central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD+ and NADH respectively.

DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as radiation can cause DNA damage, resulting in as many as 1 million individual molecular lesions per cell per day. Many of these lesions cause structural damage to the DNA molecule and can alter or eliminate the cell's ability to transcribe the gene that the affected DNA encodes. Other lesions induce potentially harmful mutations in the cell's genome, which affect the survival of its daughter cells after it undergoes mitosis. As a consequence, the DNA repair process is constantly active as it responds to damage in the DNA structure. When normal repair processes fail, and when cellular apoptosis does not occur, irreparable DNA damage may occur, including double-strand breaks and DNA crosslinkages. This can eventually lead to malignant tumors, or cancer as per the two hit hypothesis.

Poly (ADP-ribose) polymerase (PARP) is a family of proteins involved in a number of cellular processes such as DNA repair, genomic stability, and programmed cell death.

c-Jun N-terminal kinases (JNKs), were originally identified as kinases that bind and phosphorylate c-Jun on Ser-63 and Ser-73 within its transcriptional activation domain. They belong to the mitogen-activated protein kinase family, and are responsive to stress stimuli, such as cytokines, ultraviolet irradiation, heat shock, and osmotic shock. They also play a role in T cell differentiation and the cellular apoptosis pathway. Activation occurs through a dual phosphorylation of threonine (Thr) and tyrosine (Tyr) residues within a Thr-Pro-Tyr motif located in kinase subdomain VIII. Activation is carried out by two MAP kinase kinases, MKK4 and MKK7, and JNK can be inactivated by Ser/Thr and Tyr protein phosphatases. It has been suggested that this signaling pathway contributes to inflammatory responses in mammals and insects.
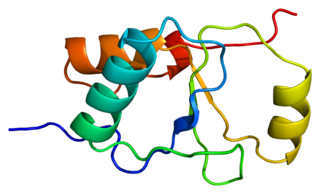
DNA repair protein XRCC1, also known as X-ray repair cross-complementing protein 1, is a protein that in humans is encoded by the XRCC1 gene. XRCC1 is involved in DNA repair, where it complexes with DNA ligase III.

ADP-ribosylation is the addition of one or more ADP-ribose moieties to a protein. It is a reversible post-translational modification that is involved in many cellular processes, including cell signaling, DNA repair, gene regulation and apoptosis. Improper ADP-ribosylation has been implicated in some forms of cancer. It is also the basis for the toxicity of bacterial compounds such as cholera toxin, diphtheria toxin, and others.

Protein C-ets-1 is a protein that in humans is encoded by the ETS1 gene. The protein encoded by this gene belongs to the ETS family of transcription factors.

Poly [ADP-ribose] polymerase 1 (PARP-1) also known as NAD+ ADP-ribosyltransferase 1 or poly[ADP-ribose] synthase 1 is an enzyme that in humans is encoded by the PARP1 gene. It is one of the PARP family of enzymes.
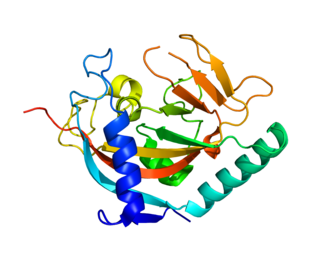
Tankyrase, also known as tankyrase 1, is an enzyme that in humans is encoded by the TNKS gene. It inhibits the binding of TERF1 to telomeric DNA.

Poly [ADP-ribose] polymerase 4 is an enzyme that in humans is encoded by the PARP4 gene.
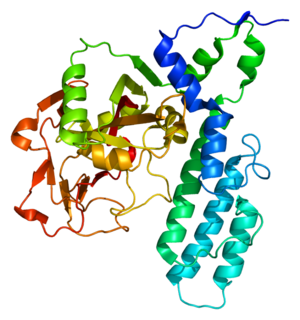
Poly [ADP-ribose] polymerase 3 is an enzyme that in humans is encoded by the PARP3 gene.

Poly [ADP-ribose] polymerase 2 is an enzyme that in humans is encoded by the PARP2 gene. It is one of the PARP family of enzymes.

Sirtuin 6 is a stress responsive protein deacetylase and mono-ADP ribosyltransferase enzyme encoded by the SIRT6 gene. SIRT6 functions in multiple molecular pathways related to aging, including DNA repair, telomere maintenance, glycolysis and inflammation.
Poly [ADP-ribose] polymerase 10 is an enzyme that in humans is encoded by the PARP10 gene.

Olaparib, sold under the brand name Lynparza, is a medication for the maintenance treatment of BRCA-mutated advanced ovarian cancer in adults. It is a PARP inhibitor, inhibiting poly ADP ribose polymerase (PARP), an enzyme involved in DNA repair. It acts against cancers in people with hereditary BRCA1 or BRCA2 mutations, which include some ovarian, breast, and prostate cancers.

PARP inhibitors are a group of pharmacological inhibitors of the enzyme poly ADP ribose polymerase (PARP).
In molecular biology, the Macro domain or A1pp domain is a module of about 180 amino acids which can bind ADP-ribose, an NAD metabolite, or related ligands. Binding to ADP-ribose can be either covalent or non-covalent: in certain cases it is believed to bind non-covalently, while in other cases it appears to bind both non-covalently through a zinc finger motif, and covalently through a separate region of the protein.
Parthanatos is a form of programmed cell death that is distinct from other cell death processes such as necrosis and apoptosis. While necrosis is caused by acute cell injury resulting in traumatic cell death and apoptosis is a highly controlled process signalled by apoptotic intracellular signals, parthanatos is caused by the accumulation of PAR and the nuclear translocation of apoptosis-inducing factor (AIF) from mitochondria. Parthanatos is also known as PARP-1 dependent cell death. PARP-1 mediates parthanatos when it is over-activated in response to extreme genomic stress and synthesizes PAR which causes nuclear translocation of AIF. Parthanatos is involved in diseases that afflict hundreds of millions of people worldwide. Well known diseases involving parthanatos include Parkinson's disease, stroke, heart attack, and diabetes. It also has potential use as a treatment for ameliorating disease and various medical conditions such as diabetes and obesity.

Talazoparib, sold under the brand name Talzenna, is an orally available poly ADP ribose polymerase (PARP) inhibitor developed by Pfizer for the treatment of advanced breast cancer with germline BRCA mutations. Talazoparib is similar to the first in class PARP inhibitor, olaparib. It was approved in October 2018, in the United States and June 2019, in the European Union for germline BRCA-mutated, HER2-negative locally advanced or metastatic breast cancer.
Ted M. Dawson is an American neurologist and neuroscientist. He is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases and Director of the Institute for Cell Engineering at Johns Hopkins University School of Medicine. He has joint appointments in the Department of Neurology, Neuroscience and Department of Pharmacology and Molecular Sciences.



















