
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to an organyl group, or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

Tartaric acid is a white, crystalline organic acid that occurs naturally in many fruits, most notably in grapes but also in tamarinds, bananas, avocados, and citrus. Its salt, potassium bitartrate, commonly known as cream of tartar, develops naturally in the process of fermentation. Potassium bitartrate is commonly mixed with sodium bicarbonate and is sold as baking powder used as a leavening agent in food preparation. The acid itself is added to foods as an antioxidant E334 and to impart its distinctive sour taste. Naturally occurring tartaric acid is a useful raw material in organic chemical synthesis. Tartaric acid, an alpha-hydroxy-carboxylic acid, is diprotic and aldaric in acid characteristics and is a dihydroxyl derivative of succinic acid.
In chemistry, an amphoteric compound is a molecule or ion that can react both as an acid and as a base. What exactly this can mean depends on which definitions of acids and bases are being used.

Phthalic anhydride is the organic compound with the formula C6H4(CO)2O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.

Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially.
Alpha hydroxy carboxylic acids, or α-hydroxy carboxylic acids (AHAs), are a group of carboxylic acids featuring a hydroxy group located one carbon atom away from the acid group. This structural aspect distinguishes them from beta hydroxy acids, where the functional groups are separated by two carbon atoms. Notable AHAs include glycolic acid, lactic acid, mandelic acid, and citric acid.
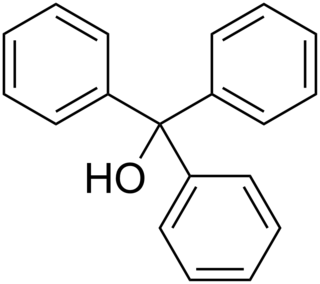
Triphenylmethanol is an organic compound. It is a white crystalline solid that is insoluble in water and petroleum ether, but well soluble in ethanol, diethyl ether, and benzene. In strongly acidic solutions, it produces an intensely yellow color, due to the formation of a stable "trityl" carbocation. Many derivatives of triphenylmethanol are important dyes.

Aconitic acid is an organic acid. The two isomers are cis-aconitic acid and trans-aconitic acid. The conjugate base of cis-aconitic acid, cis-aconitate is an intermediate in the isomerization of citrate to isocitrate in the citric acid cycle. It is acted upon by the enzyme aconitase.

Tartronic acid or 2-hydroxymalonic acid is an organic compound with the structural formula of HOHC(CO2H)2. This dicarboxylic acid is related to malonic acid. It is a white solid. It is produced by oxidation of glycerol:

Gentisic acid is a dihydroxybenzoic acid. It is a derivative of benzoic acid and a minor (1%) product of the metabolic break down of aspirin, excreted by the kidneys.

Phenylglyoxylic acid is the organic compound with the formula C6H5C(O)CO2H. The conjugate base, known as benzoylformate is the substrate of benzoylformate decarboxylase, a thiamine diphosphate-dependent enzyme:
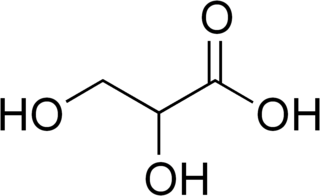
Glyceric acid refers to organic compounds with the formula HOCH2CH(OH)CO2H. It occurs naturally and is classified as three-carbon sugar acid. It is chiral. Salts and esters of glyceric acid are known as glycerates.

Phenylpyruvic acid is the organic compound with the formula C6H5CH2C(O)CO2H. It is a keto acid.

4-Hydroxyphenylacetic acid is a chemical compound found in olive oil and beer.
Mucobromic acid is an organic compound that consists of a dibrominated alkene with aldehyde and carboxylic acid functional groups. It easily tautomerizes to a furanone hemiacetal form. This compound, and the analogous mucochloric acid, form the group of known mucohalic acids. The bromide appears to behave similarly to the more heavily studied chloride.

4-Methylsalicylic acid is an organic compound with the formula CH3C6H3(CO2H)(OH). It is a white solid that is soluble in basic water and in polar organic solvents. Its functional groups include a carboxylic acid and a phenol group. It is one of four isomers of methylsalicylic acid, including the naturally occurring 6-methylsalicylic acid. The compound has few applications. It is produced by carboxylation of sodium para-cresolate: CH3C6H4ONa + CO2 → CH3C6H3(CO2Na)OH
Hydroxycarboxylic acids are carboxylic acids containing one or more hydroxy (alcohol) functional groups. They are of particular interest because several are bioactive and some are useful precursors to polyesters. The inventory is large.

6-Methylsalicylic acid is an organic compound with the formula CH3C6H3(CO2H)(OH). It is a white solid that is soluble in basic water and in polar organic solvents. At neutral pH, the acid exists as 6-methylsalicylate. Its functional groups include a carboxylic acid and a phenol group. It is one of four isomers of methylsalicylic acid.
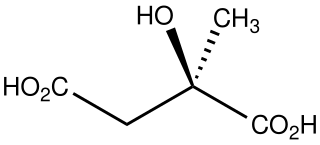
Citramalic acid is the organic compound with the formula HO2CCH2C(CH3)(OH)CO2H. A chiral compound, it is related structurally to malic acid.
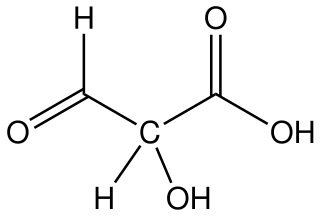
Tartronic acid semialdehyde is the organic compound with the formula OCHCH(OH)CO2H. The molecule has three functional groups, aldehyde, alcohol, and carboxylic acid. A white solid, it occurs naturally. A near neutral pH, it exists as the hydrated carboxylate (HO)2CHCH(OH)CO2−, which is referred to as tartronate semialdehyde. Tartronate semialdehyde is produced and consumed on a prodigious scale as an intermediate in photorespiration, an undesirable side reaction that competes with photosynthesis. It is produced biologically by the condensation of two equivalents of glyoxalate:
















