
In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology.
Benzonitrile is the chemical compound with the formula C6H5(CN), abbreviated PhCN. This aromatic organic compound is a colorless liquid with a sweet bitter almond odour. It is mainly used as a precursor to the resin benzoguanamine.

Benzyl alcohol (also known as α-cresol) is an aromatic alcohol with the formula C6H5CH2OH. The benzyl group is often abbreviated "Bn" (not to be confused with "Bz" which is used for benzoyl), thus benzyl alcohol is denoted as BnOH. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic odor. It is useful as a solvent for its polarity, low toxicity, and low vapor pressure. Benzyl alcohol has moderate solubility in water (4 g/100 mL) and is miscible in alcohols and diethyl ether. The anion produced by deprotonation of the alcohol group is known as benzylate or benzyloxide.

Cinnamic acid is an organic compound with the formula C6H5-CH=CH-COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a cis and a trans isomer, although the latter is more common.

Terephthalic acid is an organic compound with formula C6H4(CO2H)2. This white solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tons are produced annually. The common name is derived from the turpentine-producing tree Pistacia terebinthus and phthalic acid.

Benzoin ( or ) is an organic compound with the formula PhCH(OH)C(O)Ph. It is a hydroxy ketone attached to two phenyl groups. It appears as off-white crystals, with a light camphor-like odor. Benzoin is synthesized from benzaldehyde in the benzoin condensation. It is chiral and it exists as a pair of enantiomers: (R)-benzoin and (S)-benzoin.

Hippuric acid is a carboxylic acid and organic compound. It is found in urine and is formed from the combination of benzoic acid and glycine. Levels of hippuric acid rise with the consumption of phenolic compounds. The phenols are first converted to benzoic acid, and then to hippuric acid and excreted in urine.
In organic chemistry, a cyanohydrin reaction is an organic reaction in which an aldehyde or ketone reacts with a cyanide anion or a nitrile to form a cyanohydrin. For example:
A pinacol coupling reaction is an organic reaction in which a carbon–carbon bond is formed between the carbonyl groups of an aldehyde or a ketone in presence of an electron donor in a free radical process. The reaction product is a vicinal diol. The reaction is named after pinacol, which is the product of this reaction when done with acetone as reagent. The reaction is usually a homocoupling but intramolecular cross-coupling reactions are also possible. Pinacol was discovered by Wilhelm Rudolph Fittig in 1859.
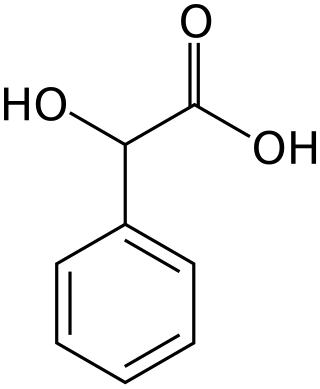
Mandelic acid is an aromatic alpha hydroxy acid with the molecular formula C6H5CH(OH)CO2H. It is a white crystalline solid that is soluble in water and polar organic solvents. It is a useful precursor to various drugs. The molecule is chiral. The racemic mixture is known as paramandelic acid.

Benzyl benzoate is an organic compound which is used as a medication and insect repellent. As a medication it is used to treat scabies and lice. For scabies either permethrin or malathion is typically preferred. It is applied to the skin as a lotion. Typically two to three applications are needed. It is also present in Balsam of Peru, Tolu balsam, and in a number of flowers.
The Étard reaction is a chemical reaction that involves the direct oxidation of an aromatic or heterocyclic bound methyl group to an aldehyde using chromyl chloride. For example, toluene can be oxidized to benzaldehyde.

Benzylamine is an organic chemical compound with the condensed structural formula C6H5CH2NH2 (sometimes abbreviated as PhCH2NH2 or BnNH2). It consists of a benzyl group, C6H5CH2, attached to an amine functional group, NH2. This colorless water-soluble liquid is a common precursor in organic chemistry and used in the industrial production of many pharmaceuticals. The hydrochloride salt was used to treat motion sickness on the Mercury-Atlas 6 mission in which NASA astronaut John Glenn became the first American to orbit the Earth.

Benzylideneacetone is the organic compound described by the formula C6H5CH=CHC(O)CH3. Although both cis- and trans-isomers are possible for the α,β-unsaturated ketone, only the trans isomer is observed. Its original preparation demonstrated the scope of condensation reactions to construct new, complex organic compounds. Benzylideneacetone is used as a flavouring ingredient in food and perfumes.

Benzilic acid is an organic compound with formula C
14H
12O
3 or (C
6H
5)2(HO)C(COOH). It is a white crystalline aromatic acid, soluble in many primary alcohols.

The Erlenmeyer–Plöchl azlactone and amino acid synthesis, named after Friedrich Gustav Carl Emil Erlenmeyer who partly discovered the reaction, is a series of chemical reactions which transform an N-acyl glycine to various other amino acids via an oxazolone.
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful.
The Petrenko-Kritschenko reaction is a classic multicomponent-name reaction that is closely related to the Robinson–Schöpf tropinone synthesis, but was published 12 years earlier.
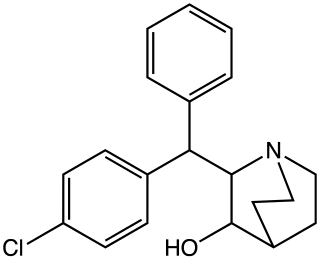
AL-1095, is a centrally acting stimulant drug with comparable effects to amphetamine, developed by Bristol in the 1970s.
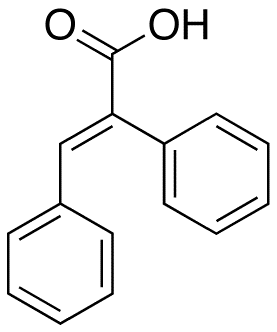
α-Phenylcinnamic acid is a phenylpropanoid, or, more specifically, a derivative of cinnamic acid. It has the formula C15H12O2 and appears as an off-white crystalline solid.















