
Telophase is the final stage in both meiosis and mitosis in a eukaryotic cell. During telophase, the effects of prophase and prometaphase are reversed. As chromosomes reach the cell poles, a nuclear envelope is re-assembled around each set of chromatids, the nucleoli reappear, and chromosomes begin to decondense back into the expanded chromatin that is present during interphase. The mitotic spindle is disassembled and remaining spindle microtubules are depolymerized. Telophase accounts for approximately 2% of the cell cycle's duration.
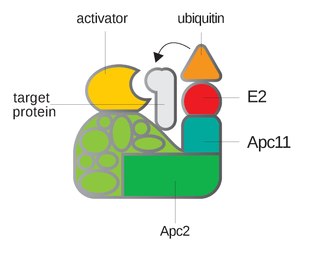
Anaphase-promoting complex is an E3 ubiquitin ligase that marks target cell cycle proteins for degradation by the 26S proteasome. The APC/C is a large complex of 11–13 subunit proteins, including a cullin (Apc2) and RING (Apc11) subunit much like SCF. Other parts of the APC/C have unknown functions but are highly conserved.
Maturation-promoting factor (abbreviated MPF, also called mitosis-promoting factor or M-Phase-promoting factor) is the cyclin-Cdk complex that was discovered first in frog eggs. It stimulates the mitotic and meiotic phases of the cell cycle. MPF promotes the entrance into mitosis (the M phase) from the G2 phase by phosphorylating multiple proteins needed during mitosis. MPF is activated at the end of G2 by a phosphatase, which removes an inhibitory phosphate group added earlier.
Endoreduplication is replication of the nuclear genome in the absence of mitosis, which leads to elevated nuclear gene content and polyploidy. Endoreplication can be understood simply as a variant form of the mitotic cell cycle (G1-S-G2-M) in which mitosis is circumvented entirely, due to modulation of cyclin-dependent kinase (CDK) activity. Examples of endoreplication characterized in arthropod, mammalian, and plant species suggest that it is a universal developmental mechanism responsible for the differentiation and morphogenesis of cell types that fulfill an array of biological functions. While endoreplication is often limited to specific cell types in animals, it is considerably more widespread in plants, such that polyploidy can be detected in the majority of plant tissues.
A spindle poison, also known as a spindle toxin, is a poison that disrupts cell division by affecting the protein threads that connect the centromere regions of chromosomes, known as spindles. Spindle poisons effectively cease the production of new cells by interrupting the mitosis phase of cell division at the spindle assembly checkpoint (SAC). However, as numerous and varied as they are, spindle poisons are not yet 100% effective at ending the formation of tumors (neoplasms). Although not 100% effective, substantive therapeutic efficacy has been found in these types of chemotherapeutic treatments. The mitotic spindle is composed of microtubules that aid, along with regulatory proteins, each other in the activity of appropriately segregating replicated chromosomes. Certain compounds affecting the mitotic spindle have proven highly effective against solid tumors and hematological malignancies.

Deubiquitinating enzymes (DUBs), also known as deubiquitinating peptidases, deubiquitinating isopeptidases, deubiquitinases, ubiquitin proteases, ubiquitin hydrolases, ubiquitin isopeptidases, are a large group of proteases that cleave ubiquitin from proteins. Ubiquitin is attached to proteins in order to regulate the degradation of proteins via the proteasome and lysosome; coordinate the cellular localisation of proteins; activate and inactivate proteins; and modulate protein-protein interactions. DUBs can reverse these effects by cleaving the peptide or isopeptide bond between ubiquitin and its substrate protein. In humans there are nearly 100 DUB genes, which can be classified into two main classes: cysteine proteases and metalloproteases. The cysteine proteases comprise ubiquitin-specific proteases (USPs), ubiquitin C-terminal hydrolases (UCHs), Machado-Josephin domain proteases (MJDs) and ovarian tumour proteases (OTU). The metalloprotease group contains only the Jab1/Mov34/Mpr1 Pad1 N-terminal+ (MPN+) (JAMM) domain proteases.

Cell cycle checkpoints are control mechanisms in the eukaryotic cell cycle which ensure its proper progression. Each checkpoint serves as a potential termination point along the cell cycle, during which the conditions of the cell are assessed, with progression through the various phases of the cell cycle occurring only when favorable conditions are met. There are many checkpoints in the cell cycle, but the three major ones are: the G1 checkpoint, also known as the Start or restriction checkpoint or Major Checkpoint; the G2/M checkpoint; and the metaphase-to-anaphase transition, also known as the spindle checkpoint. Progression through these checkpoints is largely determined by the activation of cyclin-dependent kinases by regulatory protein subunits called cyclins, different forms of which are produced at each stage of the cell cycle to control the specific events that occur therein.
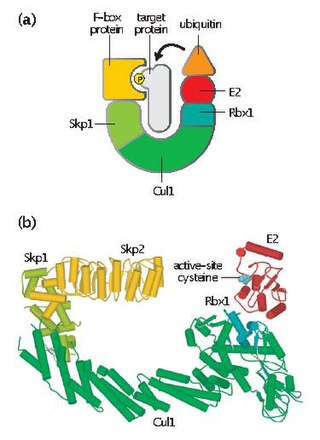
Skp, Cullin, F-box containing complex is a multi-protein E3 ubiquitin ligase complex that catalyzes the ubiquitination of proteins destined for 26S proteasomal degradation. Along with the anaphase-promoting complex, SCF has important roles in the ubiquitination of proteins involved in the cell cycle. The SCF complex also marks various other cellular proteins for destruction.
Polo-like kinases (Plks) are regulatory serine/threonin kinases of the cell cycle involved in mitotic entry, mitotic exit, spindle formation, cytokinesis, and meiosis. Only one Plk is found in the genomes of the fly Drosophila melanogaster (Polo), budding yeast (Cdc5) and fission yeast (Plo1). Vertebrates and other animals, however, have many Plk family members including Plk1, Plk2/Snk, Plk3/Prk/FnK, Plk4/Sak and Plk5. Of the vertebrate Plk family members, the mammalian Plk1 has been most extensively studied. During mitosis and cytokinesis, Plks associate with several structures including the centrosome, kinetochores, and the central spindle.

The cell division cycle protein 20 homolog is an essential regulator of cell division that is encoded by the CDC20 gene in humans. To the best of current knowledge its most important function is to activate the anaphase promoting complex (APC/C), a large 11-13 subunit complex that initiates chromatid separation and entrance into anaphase. The APC/CCdc20 protein complex has two main downstream targets. Firstly, it targets securin for destruction, enabling the eventual destruction of cohesin and thus sister chromatid separation. It also targets S and M-phase (S/M) cyclins for destruction, which inactivates S/M cyclin-dependent kinases (Cdks) and allows the cell to exit from mitosis. A closely related protein, Cdc20homologue-1 (Cdh1) plays a complementary role in the cell cycle.

Cell division cycle protein 27 homolog is a protein that in humans is encoded by the CDC27 gene.

F-box only protein 5 is a protein that in humans is encoded by the FBXO5 gene.

Cell division cycle protein 16 homolog is a protein that in humans is encoded by the CDC16 gene.

Wee1 is a nuclear kinase belonging to the Ser/Thr family of protein kinases in the fission yeast Schizosaccharomyces pombe. Wee1 has a molecular mass of 96 kDa and is a key regulator of cell cycle progression. It influences cell size by inhibiting the entry into mitosis, through inhibiting Cdk1. Wee1 has homologues in many other organisms, including mammals.
A series of biochemical switches control transitions between and within the various phases of the cell cycle. The cell cycle is a series of complex, ordered, sequential events that control how a single cell divides into two cells, and involves several different phases. The phases include the G1 and G2 phases, DNA replication or S phase, and the actual process of cell division, mitosis or M phase. During the M phase, the chromosomes separate and cytokinesis occurs.

Cdc4 is a substrate recognition component of the SCF ubiquitin ligase complex, which acts as a mediator of ubiquitin transfer to target proteins, leading to their subsequent degradation via the ubiquitin-proteasome pathway. Cdc4 targets primarily cell cycle regulators for proteolysis. It serves the function of an adaptor that brings target molecules to the core SCF complex. Cdc4 was originally identified in the model organism Saccharomyces cerevisiae. CDC4 gene function is required at G1/S and G2/M transitions during mitosis and at various stages during meiosis.
Cdc14 and Cdc14 are a gene and its protein product respectively. Cdc14 is found in most of the eukaryotes. Cdc14 was defined by Hartwell in his famous screen for loci that control the cell cycle of Saccharomyces cerevisiae. Cdc14 was later shown to encode a protein phosphatase. Cdc14 is dual-specificity, which means it has serine/threonine and tyrosine-directed activity. A preference for serines next to proline is reported. Many early studies, especially in the budding yeast Saccharomyces cerevisiae, demonstrated that the protein plays a key role in regulating late mitotic processes. However, more recent work in a range of systems suggests that its cellular function is more complex.
Clb5 and Clb6 are B-type, S-phase cyclins in yeast that assist in cell cycle regulation. Clb5 and Clb6 bind and activate Cdk1, and high levels of these cyclins are required for entering S-phase. S-phase cyclin binding to Cdk1 directly stimulates DNA replication as well as progression to the next phase of the cell cycle.
Mitotic exit is an important transition point that signifies the end of mitosis and the onset of new G1 phase for a cell, and the cell needs to rely on specific control mechanisms to ensure that once it exits mitosis, it never returns to mitosis until it has gone through G1, S, and G2 phases and passed all the necessary checkpoints. Many factors including cyclins, cyclin-dependent kinases (CDKs), ubiquitin ligases, inhibitors of cyclin-dependent kinases, and reversible phosphorylations regulate mitotic exit to ensure that cell cycle events occur in correct order with fewest errors. The end of mitosis is characterized by spindle breakdown, shortened kinetochore microtubules, and pronounced outgrowth of astral (non-kinetochore) microtubules. For a normal eukaryotic cell, mitotic exit is irreversible.

The anaphase- promoting complex or cyclosome (APC/C) is a highly specific ubiquitin protein ligase responsible for triggering events of late mitosis. In early mitosis, Cdc20 levels rise and APC/C binds to form active APC/CCdc20. This then leads to the destruction of mitotic cyclins, securin, and other proteins to trigger chromosome separation in anaphase. In early anaphase, Cdk1 is inactivated, leading to the activation of Cdh1, the other activator subunit of APC/C. This then triggers the degradation of Cdc20 and leads to the activation of APC/CCdh1 through G1 to suppress S- phase cyclin-Cdk activity. At the end of G1, APC/CCdh1 is inactivated and S- phase and mitotic cyclins gets reaccumulate as the cell progresses to S phase.













