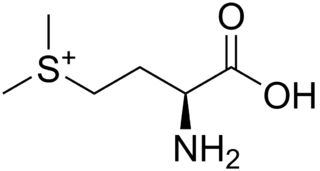
S-Methylmethionine (SMM) is a derivative of methionine with the chemical formula (CH3)2S+CH2CH2CH(NH3+)CO2−. This cation is a naturally-occurring intermediate in many biosynthetic pathways owing to the sulfonium functional group. It is biosynthesized from L-methionine and S-adenosylmethionine by the enzyme methionine S-methyltransferase. S-methylmethionine is particularly abundant in plants, being more abundant than methionine.
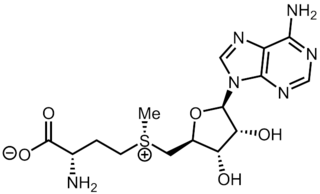
S-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952.

Hypermethioninemia is an excess of the amino acid methionine, in the blood. This condition can occur when methionine is not broken down properly in the body.
In enzymology, a homocysteine S-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an inositol 1-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an inositol 3-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, an isobutyraldoxime O-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a methionine S-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a tryptophan 2-C-methyltransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a [formate-C-acetyltransferase]-activating enzyme is an enzyme that catalyzes the chemical reaction
In enzymology, an adenosylhomocysteine nucleosidase (EC 3.2.2.9) is an enzyme that catalyzes the chemical reaction
In enzymology, a methylthioadenosine nucleosidase (EC 3.2.2.16) is an enzyme that catalyzes the chemical reaction
In enzymology, an adenosylmethionine cyclotransferase is an enzyme that catalyzes the chemical reaction
In enzymology, a discadenine synthase (EC 2.5.1.24) is an enzyme that catalyzes the chemical reaction
In enzymology, an isonocardicin synthase is an enzyme that catalyzes the chemical reaction
In enzymology, a tRNA-uridine aminocarboxypropyltransferase is an enzyme that catalyzes the chemical reaction

S-Adenosylmethionine synthetase, also known as methionine adenosyltransferase (MAT), is an enzyme that creates S-adenosylmethionine by reacting methionine and ATP.

Uroporphyrinogen-III C-methyltransferase, uroporphyrinogen methyltransferase, uroporphyrinogen-III methyltransferase, adenosylmethionine-uroporphyrinogen III methyltransferase, S-adenosyl-L-methionine-dependent uroporphyrinogen III methylase, uroporphyrinogen-III methylase, SirA, CysG, CobA, uroporphyrin-III C-methyltransferase, S-adenosyl-L-methionine:uroporphyrin-III C-methyltransferase) is an enzyme with systematic name S-adenosyl-L-methionine:uroporphyrinogen-III C-methyltransferase. This enzyme catalyses the following chemical reaction
16S rRNA (adenine1518-N6/adenine1519-N6)-dimethyltransferase (EC 2.1.1.182, S-adenosylmethionine-6-N',N'-adenosyl (rRNA) dimethyltransferase, KsgA, ksgA methyltransferase) is an enzyme with systematic name S-adenosyl-L-methionine:16S rRNA (adenine1518-N6/adenine1519-N6)-dimethyltransferase. This enzyme catalyses the following chemical reaction
Acyl-homoserine-lactone synthase is an enzyme with systematic name acyl-(acyl-carrier protein):S-adenosyl-L-methionine acyltranserase . This enzyme catalyses the following chemical reaction





