
Alkanna tinctoria, the dyer's alkanet or simply alkanet, is a herbaceous flowering plant in the borage family Boraginaceae. Its roots are used to produce a red dye. The plant is also known as dyers' bugloss, orchanet, Spanish bugloss, or Languedoc bugloss. It is native to the Mediterranean region. A. tinctoria has 30 chromosomes and is regarded as a dysploid at the tetraploid level.

Food coloring, color additive or colorant is any dye, pigment, or substance that imparts color when it is added to food or beverages. Colorants can be supplied as liquids, powders, gels, or pastes. Food coloring is commonly used in commercial products and in domestic cooking.

Thujone is a ketone and a monoterpene that occurs predominantly in two diastereomeric (epimeric) forms: (−)-α-thujone and (+)-β-thujone.
Carmine – also called cochineal, cochineal extract, crimson lake, or carmine lake – is a pigment of a bright-red color obtained from the aluminium complex derived from carminic acid. Specific code names for the pigment include natural red 4, C.I. 75470, or E120. Carmine is also a general term for a particularly deep-red color.
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully conjugated cyclic dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone". Other important examples are 1,2-benzoquinone (ortho-quinone), 1,4-naphthoquinone and 9,10-anthraquinone.

Astaxanthin is a keto-carotenoid within a group of chemical compounds known as carotenones or terpenes. Astaxanthin is a metabolite of zeaxanthin and canthaxanthin, containing both hydroxyl and ketone functional groups.

Castoreum is a yellowish exudate from the castor sacs of mature beavers and platypuses. Both animals use castoreum for various purposes; beavers use it in combination with urine to scent mark their territory, while platypuses use it in reproductive communication.

Propylparaben is the n-propyl ester of p-hydroxybenzoic acid. It occurs as a natural substance found in many plants and some insects. Additionally, it can be manufactured synthetically for use in cosmetics, pharmaceuticals, and foods. It is a member of the class of parabens and can be used as a preservative in many water-based cosmetics, such as creams, lotions, shampoos, and bath products. As a food additive, it has an E number, which is E216.
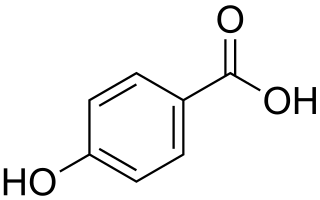
4-Hydroxybenzoic acid, also known as p-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar organic solvents such as alcohols and acetone. 4-Hydroxybenzoic acid is primarily known as the basis for the preparation of its esters, known as parabens, which are used as preservatives in cosmetics and some ophthalmic solutions. It is isomeric with 2-hydroxybenzoic acid, known as salicylic acid, a precursor to aspirin, and with 3-hydroxybenzoic acid.

Litmus is a water-soluble mixture of different dyes extracted from lichens. It is often absorbed onto filter paper to produce one of the oldest forms of pH indicator, used to test materials for acidity. In an acidic medium, blue litmus paper turns red, while in a basic or alkaline medium, red litmus paper turns blue. In short, it is a dye and indicator which is used to place substances on a pH scale.

For the parent molecule 9,10-anthraquinone, see anthraquinone

Lawsone (2-hydroxy-1,4-naphthoquinone), also known as hennotannic acid, is a red-orange dye present in the leaves of the henna plant, for which it is named, as well as in the common walnut and water hyacinth. Humans have used henna extracts containing lawsone as hair and skin dyes for more than 5,000 years. Lawsone reacts chemically with the protein keratin in skin and hair via a Michael addition reaction, resulting in a strong permanent stain that lasts until the skin or hair is shed. Darker colored staining is due to more lawsone–keratin interactions occurring, which evidently break down as the concentration of lawsone decreases and the tattoo fades. Lawsone strongly absorbs UV light, and aqueous extracts can be effective sunless tanning agents and sunscreens. Lawsone is a 1,4-naphthoquinone derivative, an analog of hydroxyquinone containing one additional ring.

Anthocyanins, also called anthocyans, are water-soluble vacuolar pigments that, depending on their pH, may appear red, purple, blue, or black. In 1835, the German pharmacist Ludwig Clamor Marquart named a chemical compound that gives flowers a blue color, Anthokyan, in his treatise "Die Farben der Blüthen". Food plants rich in anthocyanins include the blueberry, raspberry, black rice, and black soybean, among many others that are red, blue, purple, or black. Some of the colors of autumn leaves are derived from anthocyanins.
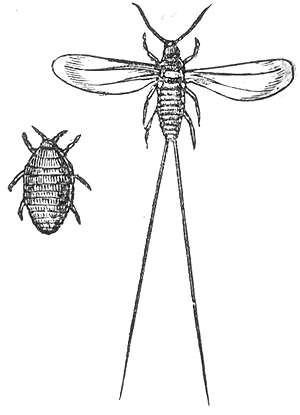
The cochineal is a scale insect in the suborder Sternorrhyncha, from which the natural dye carmine is derived. A primarily sessile parasite native to tropical and subtropical South America through North America, this insect lives on cacti in the genus Opuntia, feeding on plant moisture and nutrients. The insects are found on the pads of prickly pear cacti, collected by brushing them off the plants, and dried.

Aucubin is an iridoid glycoside. Iridoids are commonly found in plants and function as defensive compounds. Iridoids decrease the growth rates of many generalist herbivores.

Chrysanthemin is an anthocyanin. It is the 3-glucoside of cyanidin (kuromanin).

Dyeing is the craft of imparting colors to textiles in loose fiber, yarn, cloth or garment form by treatment with a dye. Archaeologists have found evidence of textile dyeing with natural dyes dating back to the Neolithic period. In China, dyeing with plants, barks and insects has been traced back more than 5,000 years. Natural insect dyes such as Tyrian purple and kermes and plant-based dyes such as woad, indigo and madder were important elements of the economies of Asia and Europe until the discovery of man-made synthetic dyes in the mid-19th century. Synthetic dyes quickly superseded natural dyes for the large-scale commercial textile production enabled by the Industrial Revolution, but natural dyes remained in use by traditional cultures around the world.
4-hydroxybenzoate geranyltransferase is an enzyme with systematic name geranyl-diphosphate:4-hydroxybenzoate 3-geranyltransferase. This enzyme catalyses the following chemical reaction

Lithospermum erythrorhizon, commonly called purple gromwell, red stoneroot, red gromwell, red-root gromwell and redroot lithospermum, is a plant species in the family Boraginaceae. It is called zǐcǎo (紫草) in Chinese, jichi (지치) in Korean, and murasaki in Japanese.

Hormuzakia aggregata is a flowering annual plant in the borage family, known by the common names massed alkanet, Arabic: لسان الثور, and Hebrew: לשון-שור מגובבת.


















