| |||
| Names | |||
|---|---|---|---|
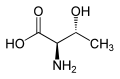
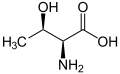
| Other names (2S,3S)-2-amino-3-hydroxybutanoic acid | |||
| Identifiers | |||
| |||
3D model (JSmol) |
| ||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider | |||
| EC Number |
| ||
| KEGG |
| ||
PubChem CID |
| ||
| UNII |
| ||
CompTox Dashboard (EPA) |
| ||
| |||
| |||
| Properties | |||
| C4H9NO3 | |||
| Molar mass | 119.120 g·mol−1 | ||
| Appearance | White solid | ||
| Melting point | 273.5–275.0 °C (524.3–527.0 °F; 546.6–548.1 K) decomposition | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Allothreonine is an amino acid with the formula CH3CH(OH)CH(NH2)CO2H. It is the diastereomer of the amino acid threonine. Like most other amino acids, allothreonine is a water-soluble colorless solid. Although not one of the proteinogenic amino acids, it has often been the subject for the synthesis of novel proteins using an expanded genetic code. [1] Racemic allothreonine can be produced in the laboratory from bromomethoxybutyric acid. [2]