Related Research Articles

Lactobacillus is a genus of Gram-positive, aerotolerant anaerobes or microaerophilic, rod-shaped, non-spore-forming bacteria. Until 2020, the genus Lactobacillus comprised over 260 phylogenetically, ecologically, and metabolically diverse species; a taxonomic revision of the genus assigned lactobacilli to 25 genera.

The human microbiome is the aggregate of all microbiota that reside on or within human tissues and biofluids along with the corresponding anatomical sites in which they reside, including the skin, mammary glands, seminal fluid, uterus, ovarian follicles, lung, saliva, oral mucosa, conjunctiva, biliary tract, and gastrointestinal tract. Types of human microbiota include bacteria, archaea, fungi, protists and viruses. Though micro-animals can also live on the human body, they are typically excluded from this definition. In the context of genomics, the term human microbiome is sometimes used to refer to the collective genomes of resident microorganisms; however, the term human metagenome has the same meaning.

Gutmicrobiota are the microorganisms, including bacteria and archaea, that live in the digestive tracts of vertebrates including humans, and of insects. Alternative terms include gutflora and gutmicrobiome. The gastrointestinal metagenome is the aggregate of all the genomes of gut microbiota. In the human, the gut is the main location of human microbiota. The gut microbiota has broad impacts, including effects on colonization, resistance to pathogens, maintaining the intestinal epithelium, metabolizing dietary and pharmaceutical compounds, controlling immune function, and even behavior through the gut-brain axis.

Bacteroides is a genus of Gram-negative, obligate anaerobic bacteria. Bacteroides species are non endospore-forming bacilli, and may be either motile or nonmotile, depending on the species. The DNA base composition is 40–48% GC. Unusual in bacterial organisms, Bacteroides membranes contain sphingolipids. They also contain meso-diaminopimelic acid in their peptidoglycan layer.
Dysbiosis is characterized by a disruption to the microbiome resulting in an imbalance in the microbiota, changes in their functional composition and metabolic activities, or a shift in their local distribution. For example, a part of the human microbiota such as the skin flora, gut flora, or vaginal flora, can become deranged, with normally dominating species underrepresented and normally outcompeted or contained species increasing to fill the void. Dysbiosis is most commonly reported as a condition in the gastrointestinal tract, particularly during small intestinal bacterial overgrowth (SIBO) or small intestinal fungal overgrowth (SIFO).
Exogenous bacteria are microorganisms introduced to closed biological systems from the external world. They exist in aquatic and terrestrial environments, as well as the atmosphere. Microorganisms in the external environment have existed on Earth for 3.5 billion years. Exogenous bacteria can be either benign or pathogenic. Pathogenic exogenous bacteria can enter a closed biological system and cause disease such as Cholera, which is induced by a waterborne microbe that infects the human intestine. Exogenous bacteria can be introduced into a closed ecosystem as well, and have mutualistic benefits for both the microbe and the host. A prominent example of this concept is bacterial flora, which consists of exogenous bacteria ingested and endogenously colonized during the early stages of life. Bacteria that are part of normal internal ecosystems, also known as bacterial flora, are called Endogenous Bacteria. A significant amount of prominent diseases are induced by exogenous bacteria such as gonorrhea, meningitis, tetanus, and syphilis. Pathogenic exogenous bacteria can enter a host via cutaneous transmission, inhalation, and consumption.

Vaginal flora, vaginal microbiota or vaginal microbiome are the microorganisms that colonize the vagina. They were discovered by the German gynecologist Albert Döderlein in 1892 and are part of the overall human flora. The amount and type of bacteria present have significant implications for an individual's overall health. The primary colonizing bacteria of a healthy individual are of the genus Lactobacillus, such as L. crispatus, and the lactic acid they produce is thought to protect against infection by pathogenic species.
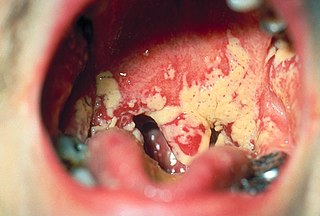
Oral microbiology is the study of the microorganisms (microbiota) of the oral cavity and their interactions between oral microorganisms or with the host. The environment present in the human mouth is suited to the growth of characteristic microorganisms found there. It provides a source of water and nutrients, as well as a moderate temperature. Resident microbes of the mouth adhere to the teeth and gums to resist mechanical flushing from the mouth to stomach where acid-sensitive microbes are destroyed by hydrochloric acid.
Jeffrey I. Gordon is a biologist and the Dr. Robert J. Glaser Distinguished University Professor and Director of the Center for Genome Sciences and Systems Biology at Washington University in St. Louis. He is internationally known for his research on gastrointestinal development and how gut microbial communities affect normal intestinal function, shape various aspects of human physiology including our nutritional status, and affect predisposition to diseases. He is a member of the National Academy of Sciences, the American Academy of Arts and Sciences, the Institute of Medicine of the National Academies, and the American Philosophical Society.
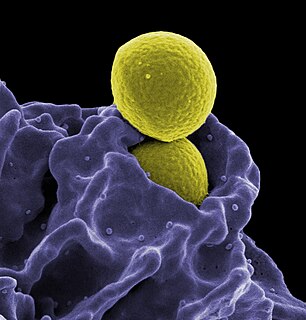
Long-term close-knit interactions between symbiotic microbes and their host can alter host immune system responses to other microorganisms, including pathogens, and are required to maintain proper homeostasis. The immune system is a host defense system consisting of anatomical physical barriers as well as physiological and cellular responses, which protect the host against harmful microorganisms while limiting host responses to harmless symbionts. Humans are home to 1013 to 1014 bacteria, roughly equivalent to the number of human cells, and while these bacteria can be pathogenic to their host most of them are mutually beneficial to both the host and bacteria.

Microbiota are the range of microorganisms that may be commensal, symbiotic, or pathogenic found in and on all multicellular organisms, including plants. Microbiota include bacteria, archaea, protists, fungi, and viruses, and have been found to be crucial for immunologic, hormonal, and metabolic homeostasis of their host.
Clostridium cadaveris is an enteric, gas-forming, motile, strictly anaerobic gram-positive bacterium of the genus Clostridium. First described by Klein in 1899, it was noted to be the most prominent bacteria during human decomposition; historically it was described as "putrefying flora".
The host–pathogen interaction is defined as how microbes or viruses sustain themselves within host organisms on a molecular, cellular, organismal or population level. This term is most commonly used to refer to disease-causing microorganisms although they may not cause illness in all hosts. Because of this, the definition has been expanded to how known pathogens survive within their host, whether they cause disease or not.

The gut–brain axis is the two-way biochemical signaling that takes place between the gastrointestinal tract and the central nervous system (CNS). The term "gut–brain axis" is occasionally used to refer to the role of the gut microbiota in the interplay as well. The "microbiota–gut–brainaxis" explicitly includes the role of gut microbiota in the biochemical signaling events that take place between the GI tract and the CNS. Broadly defined, the gut–brain axis includes the central nervous system, neuroendocrine system, neuroimmune systems, the hypothalamic–pituitary–adrenal axis, sympathetic and parasympathetic arms of the autonomic nervous system, the enteric nervous system, vagus nerve, and the gut microbiota.
Bacteriotherapy is the purposeful use of bacteria or their products in treating an illness. Forms of bacteriotherapy include the use of probiotics, microorganisms that provide health benefits when consumed; fecal matter transplants (FMT) /intestinal microbiota transplant (IMT), the transfer of gut microorganisms from the fecal matter of healthy donors to recipient patients to restore microbiota; or synbiotics which combine prebiotics, indigestible ingredients that promote growth of beneficial microorganisms, and probiotics. Through these methods, the gut microbiota, the community of 300-500 microorganism species that live in the digestive tract of animals aiding in digestion, energy storage, immune function and protection against pathogens, can be recolonized with favorable bacteria, which in turn has therapeutic effects.
The initial acquisition of microbiota is the formation of an organism's microbiota immediately before and after birth. The microbiota are all the microorganisms including bacteria, archaea and fungi that colonize the organism. The microbiome is another term for microbiota or can refer to the collected genomes.
The microbiota describes the sum of all symbiotic microorganisms living on or in an organism. The fruit fly Drosophila melanogaster is a model organism and known as one of the most investigated organisms worldwide. The microbiota in flies is less complex than that found in humans. It still has an influence on the fitness of the fly, and it affects different life-history characteristics such as lifespan, resistance against pathogens (immunity) and metabolic processes (digestion). Considering the comprehensive toolkit available for research in Drosophila, analysis of its microbiome could enhance our understanding of similar processes in other types of host-microbiota interactions, including those involving humans. Microbiota plays key roles in the intestinal immune and metabolic responses via their fermentation product, acetate.
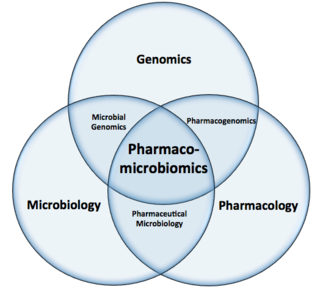
Pharmacomicrobiomics, first proposed by Prof. Marco Candela for the ERC-2009-StG project call and later publicly used in 2010, is defined as the effect of microbiome variations on drug disposition, action, and toxicity. Pharmacomicrobiomics is concerned with the interaction between xenobiotics, or foreign compounds, and the gut microbiome. It is estimated that over 100 trillion prokaryotes representing more than 1000 species reside in the gut. Within the gut, microbes help modulate developmental, immunological and nutrition host functions. The aggregate genome of microbes extends the metabolic capabilities of humans, allowing them to capture nutrients from diverse sources. Namely, through the secretion of enzymes that assist in the metabolism of chemicals foreign to the body, modification of liver and intestinal enzymes, and modulation of the expression of human metabolic genes, microbes can significantly impact the ingestion of xenobiotics.
The human milk microbiota, also known as human milk probiotics (HMP), refers to the microbiota residing in the human mammary glands and breast milk. Human breast milk has been traditionally assumed to be sterile, but more recently both microbial culture and culture-independent techniques have confirmed that human milk contains diverse communities of bacteria which are distinct from other microbial communities inhabiting the human body.

Snodgrassella alvi is a species of Gram-negative bacteria within the Neisseriaceae and the only known species of the genus Snodgrassella. It was isolated and scientifically described in 2012 by Waldan K. Kwong and Nancy A. Moran, who named the bacteria after the American entomologist Robert Evans Snodgrass.
References
- ↑ [Fox, J., Anderson, L., Loew, F. and Quimby F. Laboratory animal medicine. 2nd Ed. 2002. Academic Press. 46-47.]
- 1 2 3 4 5 6 7 8 9 [Fox, J., Barthold, S., Davisson, M., Newcomer, C., Quimby F. and Smith, A. The mouse in biomedical research. 2nd Ed. 2007. Elsevier, Inc. 227-229.]
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 [Dewhirst, F., Chien, C.-C., Paster, B., Ericson, R., Orcutt, R., Schauer, D., and Fox, J. Phylogeny of the defined murine microbiota: altered Schaedler flora. 1999. Appl. Environ, Microbiol. 65(8):3287.]
- 1 2 [Guarner, F., Malagelada, J. R. Review: gut flora in health and disease. 2003. The Lancet. 361(9356):512-219.]
- 1 2 3 4 5 6 [Fox, J., Anderson, L., Loew, F. and Quimby F. Laboratory animal medicine. 2nd Ed. 2002. Academic Press. 46-47.]
- 1 2 3 [Sarma-Rupavtarm, R., Ge, Z., Schauer, D., Fox, J., and Polz, M. Spatial distribution and stability of the eight microbial species of the altered Schaedler flora in the mouse gastrointestinal tract. 2004. Appl. Environ. Microbiol. 70(5):2791.]
- 1 2 3 [Stehr, M., Greweling, M., Tischer, S., Singh, M., Blöcker, H., Monner, D., and Müller, W. Charles River altered Schaedler flora (CRASF ®) remained stable for four years in a mouse colony housed in individually ventilated cages. 2009. Lab Anim. 43:362.]
- 1 2 3 [Ge, Z., Feng, Y., Taylor, N., Ohtani, M., Polz, M., Schauer, D., and Fox, J. Colonization dynamics of altered Schaedler flora is influenced by gender, aging, and Helicobacter hepaticus infection in the intestines of Swiss Webster mice. 2006. Appl. Environ, Microbiol. 72(7):5100.]
- 1 2 [Alexander, A., Orcutt, R., Henry, J., Baker, J., Bissahoyo, A., and Threadgill, D. Quantitative PCR assays for mouse enteric flora reveal strain-dependent differences in composition that are influenced by the microenvironment. 2006. Mammalian Genome. 17(11):1093-1104. ]
- ↑ [Bouskra, D., Brézillon, C., Bérard, M., Werts, C., Varona, R., Boneca, IG., and Eberl, G. lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. 2008. Nature. 456(7221):507-510.]
- ↑ [Geuking, M., Cahenzli, J., Lawson, M., Ng, D., Slack, E., Hapfelmeier, S., McCoy, K., and Macpherson, A. Intestinal bacterial colonization induces mutualistic regulatory T cell response. 2011. Immunity. 34:794-806.]
- ↑ [M. T. Whary, S. J. Danon, Y. Feng, Z. Ge, N. Sundina, V. Ng, N. S. Taylor, A. B. Rogers and J. G. Fox. Rapid onset of ulcerative typhlocolitis in B6.129P2-IL10tm1Cgn (IL-10-/-) mice infected with Helicobacter trogontum is associated with decreased colonization by the altered Schaedler flora. 2006. Infect. Immun. 74(12):6615.]
- ↑ [Fox, J. G. Helicobacter bilis: bacterial provocateur orchestrates host immune responses to commensal flora in a model of inflammatory bowel disease. 2007. Gut. 56:898-900.]
- ↑ [Kane, M., Case, L., Kopaskie, K., Kozlova, A., MacDearmid, C., Chervonsky, A., and Golovkina, T. Successful transmission of a retrovirus depends on the commensal microbiota. 2011. Science. 334(6053):245-249.]
- ↑ [Rohde, C., Wells, D., Robosky, L, Manning, M., Clifford, C., Reily, M., and Robertson, D. Metabonomic evaluation of Schaedler altered microflora rats. 2007. Chem. Res. Toxicol. 20:1388-1392]