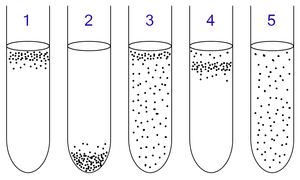
An obligate aerobe is an organism that requires oxygen to grow. Through cellular respiration, these organisms use oxygen to metabolise substances, like sugars or fats, to obtain energy. In this type of respiration, oxygen serves as the terminal electron acceptor for the electron transport chain. Aerobic respiration has the advantage of yielding more energy than fermentation or anaerobic respiration, but obligate aerobes are subject to high levels of oxidative stress.

An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. The electrons that transferred from NADH and FADH2 to the ETC involves 4 multi-subunit large enzymes complexes and 2 mobile electron carriers. Many of the enzymes in the electron transport chain are membrane-bound.

An aerobic organism or aerobe is an organism that can survive and grow in an oxygenated environment. In contrast, an anaerobic organism (anaerobe) is any organism that does not require oxygen for growth. Some anaerobes react negatively or even die if oxygen is present. The ability to exhibit aerobic respiration may yield benefits to the aerobic organism, as aerobic respiration yields more energy than anaerobic respiration. In July 2020, marine biologists reported that aerobic microorganisms (mainly), in "quasi-suspended animation", were found in organically-poor sediments, up to 101.5 million years old, 250 feet below the seafloor in the South Pacific Gyre (SPG), and could be the longest-living life forms ever found.
An anaerobic organism or anaerobe is any organism that does not require molecular oxygen for growth. It may react negatively or even die if free oxygen is present. In contrast, an aerobic organism (aerobe) is an organism that requires an oxygenated environment. Anaerobes may be unicellular or multicellular. Most fungi are obligate aerobes, requiring oxygen to survive. However, some species, such as the Chytridiomycota that reside in the rumen of cattle, are obligate anaerobes; for these species, anaerobic respiration is used because oxygen will disrupt their metabolism or kill them. Deep waters of the ocean are a common anoxic environment.

Clostridium is a genus of anaerobic, Gram-positive bacterium. Species of Clostridium inhabit soils and the intestinal tract of animals, including humans. This genus includes several significant human pathogens, including the causative agents of botulism and tetanus. It also formerly included an important cause of diarrhea, Clostridioides difficile, which was reclassified into the Clostridioides genus in 2016.
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.

A facultative anaerobic organism is an organism that makes ATP by aerobic respiration if oxygen is present, but is capable of switching to fermentation if oxygen is absent.

A microaerophile is a microorganism that requires environments containing lower levels of dioxygen than that are present in the atmosphere (i.e. < 21% O2; typically 2–10% O2) for optimal growth. A more restrictive interpretation requires the microorganism to be obligate in this requirement. Many microaerophiles are also capnophiles, requiring an elevated concentration of carbon dioxide (e.g. 10% CO2 in the case of Campylobacter species).
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can be applied to any desymmetrizing reaction of the following type, regardless of whether it is a redox or some other type of process:
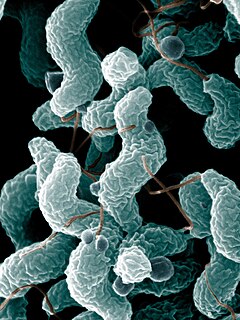
Capnophiles are microorganisms that thrive in the presence of high concentrations of carbon dioxide.

Aerotolerant anaerobes use fermentation to produce ATP. They do not use oxygen, but they can protect themselves from reactive oxygen molecules. In contrast, obligate anaerobes can be harmed by reactive oxygen molecules.

Bacteroides is a genus of Gram-negative, obligate anaerobic bacteria. Bacteroides species are non endospore-forming bacilli, and may be either motile or nonmotile, depending on the species. The DNA base composition is 40–48% GC. Unusual in bacterial organisms, Bacteroides membranes contain sphingolipids. They also contain meso-diaminopimelic acid in their peptidoglycan layer.

Bacteroides fragilis is an anaerobic, Gram-negative, pleomorphic to rod-shaped bacterium. It is part of the normal microbiota of the human colon and is generally commensal, but can cause infection if displaced into the bloodstream or surrounding tissue following surgery, disease, or trauma.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.
The Pasteur effect describes how available oxygen inhibits ethanol fermentation, driving yeast to switch toward aerobic respiration for increased generation of the energy carrier adenosine triphosphate (ATP).
Hydrogen-oxidizing bacteria are a group of facultative autotrophs that can use hydrogen as an electron donor. They can be divided into aerobes and anaerobes. The former use hydrogen as an electron donor and oxygen as an acceptor while the latter use sulphate or nitrogen dioxide as electron acceptors. Species of both types have been isolated from a variety of environments, including fresh waters, sediments, soils, activated sludge, hot springs, hydrothermal vents and percolating water.
Geobacillus thermoglucosidasius is a thermophilic gram-positive bacterium, and a member of the Bacillota phylum. It was first isolated from soil in Japan in 1983.

Fumarate reductase (quinol) is an enzyme with systematic name succinate:quinone oxidoreductase. This enzyme catalyzes the following chemical reaction:

Nickel superoxide dismutase (Ni-SOD) is a metalloenzyme that, like the other superoxide dismutases, protects cells from oxidative damage by catalyzing the disproportionation of the cytotoxic superoxide radical to hydrogen peroxide and molecular oxygen. Superoxide is a reactive oxygen species that is produced in large amounts during photosynthesis and aerobic cellular respiration. The equation for the disproportionation of superoxide is shown below:
Dissimilatory metal-reducing microorganisms are a group of microorganisms (both bacteria and archaea) that can perform anaerobic respiration utilizing a metal as terminal electron acceptor rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration. The most common metals used for this end are iron [Fe(III)] and manganese [Mn(IV)], which are reduced to Fe(II) and Mn(II) respectively, and most microorganisms that reduce Fe(III) can reduce Mn(IV) as well. But other metals and metalloids are also used as terminal electron acceptors, such as vanadium [V(V)], chromium [Cr(VI)], molybdenum [Mo(VI)], cobalt [Co(III)], palladium [Pd(II)], gold [Au(III)], and mercury [Hg(II)].