
The large intestine, also known as the large bowel, is the last part of the gastrointestinal tract and of the digestive system in tetrapods. Water is absorbed here and the remaining waste material is stored in the rectum as feces before being removed by defecation. The colon is the longest portion of the large intestine, and the terms are often used interchangeably but most sources define the large intestine as the combination of the cecum, colon, rectum, and anal canal. Some other sources exclude the anal canal.

The human microbiome is the aggregate of all microbiota that reside on or within human tissues and biofluids along with the corresponding anatomical sites in which they reside, including the gastrointestinal tract, skin, mammary glands, seminal fluid, uterus, ovarian follicles, lung, saliva, oral mucosa, conjunctiva, and the biliary tract. Types of human microbiota include bacteria, archaea, fungi, protists, and viruses. Though micro-animals can also live on the human body, they are typically excluded from this definition. In the context of genomics, the term human microbiome is sometimes used to refer to the collective genomes of resident microorganisms; however, the term human metagenome has the same meaning.

Gut microbiota, gut microbiome, or gut flora are the microorganisms, including bacteria, archaea, fungi, and viruses, that live in the digestive tracts of animals. The gastrointestinal metagenome is the aggregate of all the genomes of the gut microbiota. The gut is the main location of the human microbiome. The gut microbiota has broad impacts, including effects on colonization, resistance to pathogens, maintaining the intestinal epithelium, metabolizing dietary and pharmaceutical compounds, controlling immune function, and even behavior through the gut–brain axis.

Bacteroides is a genus of Gram-negative, obligate anaerobic bacteria. Bacteroides species are non endospore-forming bacilli, and may be either motile or nonmotile, depending on the species. The DNA base composition is 40–48% GC. Unusual in bacterial organisms, Bacteroides membranes contain sphingolipids. They also contain meso-diaminopimelic acid in their peptidoglycan layer.

Bacteroides fragilis is an anaerobic, Gram-negative, pleomorphic to rod-shaped bacterium. It is part of the normal microbiota of the human colon and is generally commensal, but can cause infection if displaced into the bloodstream or surrounding tissue following surgery, disease, or trauma.
Dysbiosis is characterized by a disruption to the microbiome resulting in an imbalance in the microbiota, changes in their functional composition and metabolic activities, or a shift in their local distribution. For example, a part of the human microbiota such as the skin flora, gut flora, or vaginal flora, can become deranged, with normally dominating species underrepresented and normally outcompeted or contained species increasing to fill the void. Similar to the human gut microbiome, diverse microbes colonize the plant rhizosphere, and dysbiosis in the rhizosphere, can negatively impact plant health. Dysbiosis is most commonly reported as a condition in the gastrointestinal tract or plant rhizosphere.
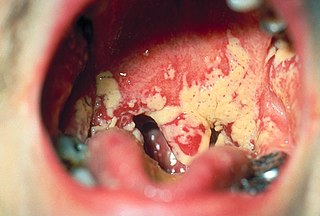
Oral microbiology is the study of the microorganisms (microbiota) of the oral cavity and their interactions between oral microorganisms or with the host. The environment present in the human mouth is suited to the growth of characteristic microorganisms found there. It provides a source of water and nutrients, as well as a moderate temperature. Resident microbes of the mouth adhere to the teeth and gums to resist mechanical flushing from the mouth to stomach where acid-sensitive microbes are destroyed by hydrochloric acid.
Methanobrevibacter smithii is the predominant archaeon in the microbiota of the human gut. M. smithii has a coccobacillus shape. It plays an important role in the efficient digestion of polysaccharides by consuming the end products of bacterial fermentation. Methanobrevibacter smithii is a single-celled microorganism from the Archaea domain. M. smithii is a methanogen, and a hydrogenotroph that recycles the hydrogen by combining it with carbon dioxide to methane. The removal of hydrogen by M. smithii is thought to allow an increase in the extraction of energy from nutrients by shifting bacterial fermentation to more oxidized end products.

Long-term close-knit interactions between symbiotic microbes and their host can alter host immune system responses to other microorganisms, including pathogens, and are required to maintain proper homeostasis. The immune system is a host defense system consisting of anatomical physical barriers as well as physiological and cellular responses, which protect the host against harmful microorganisms while limiting host responses to harmless symbionts. Humans are home to 1013 to 1014 bacteria, roughly equivalent to the number of human cells, and while these bacteria can be pathogenic to their host most of them are mutually beneficial to both the host and bacteria.

Microbiota are the range of microorganisms that may be commensal, mutualistic, or pathogenic found in and on all multicellular organisms, including plants. Microbiota include bacteria, archaea, protists, fungi, and viruses, and have been found to be crucial for immunologic, hormonal, and metabolic homeostasis of their host.
Faecalibacterium is a genus of bacteria. The genus contains several species including Faecalibacterium prausnitzii, Faecalibacterium butyricigenerans, Faecalibacterium longum, Faecalibacterium duncaniae, Faecalibacterium hattorii, and Faecalibacterium gallinarum. Its first known species, Faecalibacterium prausnitzii is gram-positive, mesophilic, rod-shaped, and anaerobic, and is one of the most abundant and important commensal bacteria of the human gut microbiota. It is non-spore forming and non-motile. These bacteria produce butyrate and other short-chain fatty acids through the fermentation of dietary fiber. The production of butyrate makes them an important member of the gut microbiota, fighting against inflammation.
Prevotella is a genus of Gram-negative bacteria.
Beta porphyranase is an enzyme responsible for the degradation of porphyran, which composes the cell wall of red algae. So far only five β-porphyranases have been identified: PorA and PorB are found in the marine bacteria Zobellia galactinovirans. A wild-type porphyranase activity has been found in Pseudoalteromonas atlantica. BpGH16B and BpGH86A have been found in the human gut bacterium, Bacteroides plebeius, of Japanese individuals.
Microbiota-accessible carbohydrates (MACs) are carbohydrates that are resistant to digestion by a host's metabolism, and are made available for gut microbes, as prebiotics, to ferment or metabolize into beneficial compounds, such as short chain fatty acids. The term, ‘‘microbiota-accessible carbohydrate’’ contributes to a conceptual framework for investigating and discussing the amount of metabolic activity that a specific food or carbohydrate can contribute to a host's microbiota.
Sarkis Mazmanian is an American medical microbiologist who has served as a professor at the California Institute of Technology since 2006. He is currently the Luis & Nelly Soux Professor of Microbiology in the Division of Biology and Biological Engineering, and a board member of Seed. Prior to this, Mazmanian was affiliated with Harvard Medical School and the University of Chicago. In 2012, Mazmanian was awarded a MacArthur Fellowship for his pioneering work on the human microbiome.
The microbiota are the sum of all symbiotic microorganisms living on or in an organism. The fruit fly Drosophila melanogaster is a model organism and known as one of the most investigated organisms worldwide. The microbiota in flies is less complex than that found in humans. It still has an influence on the fitness of the fly, and it affects different life-history characteristics such as lifespan, resistance against pathogens (immunity) and metabolic processes (digestion). Considering the comprehensive toolkit available for research in Drosophila, analysis of its microbiome could enhance our understanding of similar processes in other types of host-microbiota interactions, including those involving humans. Microbiota plays key roles in the intestinal immune and metabolic responses via their fermentation product, acetate.
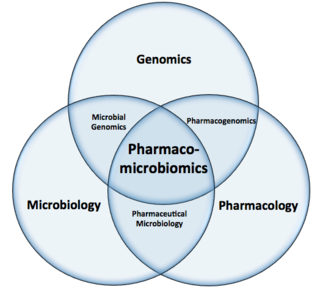
Pharmacomicrobiomics, proposed by Prof. Marco Candela for the ERC-2009-StG project call, and publicly coined for the first time in 2010 by Rizkallah et al., is defined as the effect of microbiome variations on drug disposition, action, and toxicity. Pharmacomicrobiomics is concerned with the interaction between xenobiotics, or foreign compounds, and the gut microbiome. It is estimated that over 100 trillion prokaryotes representing more than 1000 species reside in the gut. Within the gut, microbes help modulate developmental, immunological and nutrition host functions. The aggregate genome of microbes extends the metabolic capabilities of humans, allowing them to capture nutrients from diverse sources. Namely, through the secretion of enzymes that assist in the metabolism of chemicals foreign to the body, modification of liver and intestinal enzymes, and modulation of the expression of human metabolic genes, microbes can significantly impact the ingestion of xenobiotics.
The human milk microbiota, also known as human milk probiotics (HMP), encompasses the microbiota–the community of microorganisms–present within the human mammary glands and breast milk. Contrary to the traditional belief that human breast milk is sterile, advancements in both microbial culture and culture-independent methods have confirmed that human milk harbors diverse communities of bacteria. These communities are distinct in composition from other microbial populations found within the human body which constitute the human microbiome.
Phocaeicola plebeius, formerly Bacteroides plebeius, is a microbe found in the human gut, most often found in Japan natives. It is able to digest porphyran, a polysacchide from Porphyra seaweed (nori) that humans cannot digest on their own. The porphyranase-encoding gene Bp1689 is believed to have been derived from the microbe Zobellia galactanivorans via horizontal gene transfer, as part of a gene cluster containing other carbohydrate-active enzymes.

Phocaeicola vulgatus,, is a mutualistic anaerobic Gram negative rod bacteria commonly found in the human gut microbiome and isolated from feces. P. vulgatus has medical relevance and has been notable in scientific research due to its production of fatty acids, potential use as a probiotic, and associations with protecting against and worsening some inflammatory diseases. Due to the difficulties in culturing anaerobic bacteria, P. vulgatus is still highly uncharacterised so efforts are being made to make use of multi-omic approaches to investigate the human gut microbiome more thoroughly in hopes to fully understand the role of this species in the development of and protection against diseases, as well as its potential uses in medicine and research. Generally, P. vulgatus is considered as a beneficial bacteria that contributes to digestion and a balanced microbiome, but it has been known to cause opportunistic infections and induce or worsen inflammatory responses. Due to its abundance in the microbiome, some researchers are investigating these species in hopes that it will be a suitable model organism for gut microbiome research, like Bacteroides thetaiotaomicron.










