In materials science, a metal matrix composite (MMC) is a composite material with fibers or particles dispersed in a metallic matrix, such as copper, aluminum, or steel. The secondary phase is typically a ceramic or another metal. They are typically classified according to the type of reinforcement: short discontinuous fibers (whiskers), continuous fibers, or particulates. There is some overlap between MMCs and cermets, with the latter typically consisting of less than 20% metal by volume. When at least three materials are present, it is called a hybrid composite. MMCs can have much higher strength-to-weight ratios, stiffness, and ductility than traditional materials, so they are often used in demanding applications. MMCs typically have lower thermal and electrical conductivity and poor resistance to radiation, limiting their use in the very harshest environments.
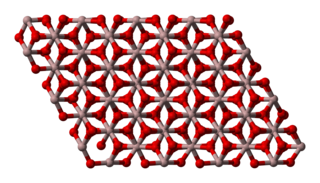
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum in various forms and applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is used to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.
In materials science, superplasticity is a state in which solid crystalline material is deformed well beyond its usual breaking point, usually over about 400% during tensile deformation. Such a state is usually achieved at high homologous temperature. Examples of superplastic materials are some fine-grained metals and ceramics. Other non-crystalline materials (amorphous) such as silica glass and polymers also deform similarly, but are not called superplastic, because they are not crystalline; rather, their deformation is often described as Newtonian fluid. Superplastically deformed material gets thinner in a very uniform manner, rather than forming a "neck" that leads to fracture. Also, the formation of microvoids, which is another cause of early fracture, is inhibited. Superplasticity must not be confused with superelasticity.

Tungsten carbide is a chemical compound containing equal parts of tungsten and carbon atoms. In its most basic form, tungsten carbide is a fine gray powder, but it can be pressed and formed into shapes through sintering for use in industrial machinery, engineering facilities, molding blocks, cutting tools, chisels, abrasives, armor-piercing bullets and jewelry.

Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts.

Titanium diboride (TiB2) is an extremely hard ceramic which has excellent heat conductivity, oxidation stability and wear resistance. TiB2 is also a reasonable electrical conductor, so it can be used as a cathode material in aluminium smelting and can be shaped by electrical discharge machining.

An intermetallic is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic elements. Intermetallics are generally hard and brittle, with good high-temperature mechanical properties. They can be classified as stoichiometric or nonstoichiometic.

Titanium nitride is an extremely hard ceramic material, often used as a physical vapor deposition (PVD) coating on titanium alloys, steel, carbide, and aluminium components to improve the substrate's surface properties.

Yttrium oxide, also known as yttria, is Y2O3. It is an air-stable, white solid substance.

A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Key characteristics of a superalloy include mechanical strength, thermal creep deformation resistance, surface stability, and corrosion and oxidation resistance.

Beryllium oxide (BeO), also known as beryllia, is an inorganic compound with the formula BeO. This colourless solid is an electrical insulator with a higher thermal conductivity than any other non-metal except diamond, and exceeds that of most metals. As an amorphous solid, beryllium oxide is white. Its high melting point leads to its use as a refractory material. It occurs in nature as the mineral bromellite. Historically and in materials science, beryllium oxide was called glucina or glucinium oxide, owing to its sweet taste.

In materials science, a metal foam is a material or structure consisting of a solid metal with gas-filled pores comprising a large portion of the volume. The pores can be sealed or interconnected. The defining characteristic of metal foams is a high porosity: typically only 5–25% of the volume is the base metal. The strength of the material is due to the square–cube law.

Zirconium carbide (ZrC) is an extremely hard refractory ceramic material, commercially used in tool bits for cutting tools. It is usually processed by sintering.

Lithium oxide (Li
2O) or lithia is an inorganic chemical compound. It is a white solid. Although not specifically important, many materials are assessed on the basis of their Li2O content. For example, the Li2O content of the principal lithium mineral spodumene (LiAlSi2O6) is 8.03%.

Thermal spraying techniques are coating processes in which melted materials are sprayed onto a surface. The "feedstock" is heated by electrical or chemical means.

Thermal barrier coatings (TBCs) are advanced materials systems usually applied to metallic surfaces on parts operating at elevated temperatures, such as gas turbine combustors and turbines, and in automotive exhaust heat management. These 100 μm to 2 mm thick coatings of thermally insulating materials serve to insulate components from large and prolonged heat loads and can sustain an appreciable temperature difference between the load-bearing alloys and the coating surface. In doing so, these coatings can allow for higher operating temperatures while limiting the thermal exposure of structural components, extending part life by reducing oxidation and thermal fatigue. In conjunction with active film cooling, TBCs permit working fluid temperatures higher than the melting point of the metal airfoil in some turbine applications. Due to increasing demand for more efficient engines running at higher temperatures with better durability/lifetime and thinner coatings to reduce parasitic mass for rotating/moving components, there is significant motivation to develop new and advanced TBCs. The material requirements of TBCs are similar to those of heat shields, although in the latter application emissivity tends to be of greater importance.
Nickel aluminide refers to either of two widely used intermetallic compounds, Ni3Al or NiAl, but the term is sometimes used to refer to any nickel–aluminium alloy. These alloys are widely used because of their high strength even at high temperature, low density, corrosion resistance, and ease of production. Ni3Al is of specific interest as a precipitate in nickel-based superalloys, where it is called the γ' (gamma prime) phase. It gives these alloys high strength and creep resistance up to 0.7–0.8 of its melting temperature. Meanwhile, NiAl displays excellent properties such as lower density and higher melting temperature than those of Ni3Al, and good thermal conductivity and oxidation resistance. These properties make it attractive for special high-temperature applications like coatings on blades in gas turbines and jet engines. However, both these alloys have the disadvantage of being quite brittle at room temperature, with Ni3Al remaining brittle at high temperatures as well. To address this problem, has been shown that Ni3Al can be made ductile when manufactured in single-crystal form rather than in polycrystalline form.
Exhaust heat management is the means of lessening the damaging or performance-robbing effects of internal combustion engine exhaust heat by preventing heat from escaping from the exhaust system and into the engine compartment on automobiles.
Iron aluminides are intermetallic compounds of iron and aluminium - they typically contain ~18% Al or more.

Detonation spraying is one of the many forms of thermal spraying techniques that are used to apply a protective coating at supersonic velocities to a material in order to change its surface characteristics. This is primarily to improve the durability of a component. It was first invented in 1955 by H.B. Sargent, R.M. Poorman and H. Lamprey and is applied to a component using a specifically designed detonation gun (D-gun). The component being sprayed must be prepared correctly by removing all surface oils, greases, debris and roughing up the surface in order to achieve a strongly bonded detonation spray coating. This process involves the highest velocities and temperatures (≈4000 °C) of coating materials compared to all other forms of thermal spraying techniques. Because of these characteristics, detonation spraying is able to apply low porous and low oxygen content protective coatings that protect against corrosion, abrasion and adhesion under low load.













