 | |
| Names | |
|---|---|
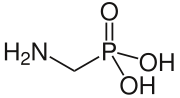
| Preferred IUPAC name (Aminomethyl)phosphonic acid | |
| Other names Aminomethanephosphonic acid | |
| Identifiers | |
3D model (JSmol) | |
| Abbreviations | AMPA; AMeP |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.152.014 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| CH6NO3P | |
| Molar mass | 111.037 g·mol−1 |
| Appearance | Solid |
| Melting point | 338 to 344 °C (640 to 651 °F; 611 to 617 K) |
| Acidity (pKa) | 0.4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Aminomethylphosphonic acid (AMPA) is a aminophosphonate with a weak phosphonic acid group.
