
An axon or nerve fiber is a long, slender projection of a nerve cell, or neuron, in vertebrates, that typically conducts electrical impulses known as action potentials away from the nerve cell body. The function of the axon is to transmit information to different neurons, muscles, and glands. In certain sensory neurons, such as those for touch and warmth, the axons are called afferent nerve fibers and the electrical impulse travels along these from the periphery to the cell body and from the cell body to the spinal cord along another branch of the same axon. Axon dysfunction can be the cause of many inherited and acquired neurological disorders that affect both the peripheral and central neurons. Nerve fibers are classed into three types – group A nerve fibers, group B nerve fibers, and group C nerve fibers. Groups A and B are myelinated, and group C are unmyelinated. These groups include both sensory fibers and motor fibers. Another classification groups only the sensory fibers as Type I, Type II, Type III, and Type IV.

Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters.

An action potential occurs when the membrane potential of a specific cell rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of excitable cells, which include animal cells like neurons and muscle cells, as well as some plant cells. Certain endocrine cells such as pancreatic beta cells, and certain cells of the anterior pituitary gland are also excitable cells.
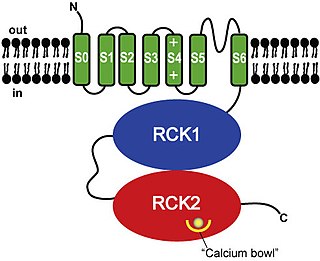
BK channels (big potassium), are large conductance calcium-activated potassium channels, also known as Maxi-K, slo1, or Kca1.1. BK channels are voltage-gated potassium channels that conduct large amounts of potassium ions (K+) across the cell membrane, hence their name, big potassium. These channels can be activated (opened) by either electrical means, or by increasing Ca2+ concentrations in the cell. BK channels help regulate physiological processes, such as circadian behavioral rhythms and neuronal excitability. BK channels are also involved in many processes in the body, as it is a ubiquitous channel. They have a tetrameric structure that is composed of a transmembrane domain, voltage sensing domain, potassium channel domain, and a cytoplasmic C-terminal domain, with many X-ray structures for reference. Their function is to repolarize the membrane potential by allowing for potassium to flow outward, in response to a depolarization or increase in calcium levels.

The axon hillock is a specialized part of the cell body of a neuron that connects to the axon. It can be identified using light microscopy from its appearance and location in a neuron and from its sparse distribution of Nissl substance.
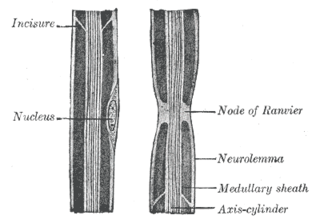
In neuroscience and anatomy, nodes of Ranvier, also known as myelin-sheath gaps, occur along a myelinated axon where the axolemma is exposed to the extracellular space. Nodes of Ranvier are uninsulated and highly enriched in ion channels, allowing them to participate in the exchange of ions required to regenerate the action potential. Nerve conduction in myelinated axons is referred to as saltatory conduction due to the manner in which the action potential seems to "jump" from one node to the next along the axon. This results in faster conduction of the action potential.
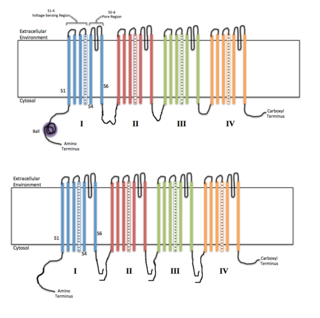
Voltage-gated ion channels are a class of transmembrane proteins that form ion channels that are activated by changes in the electrical membrane potential near the channel. The membrane potential alters the conformation of the channel proteins, regulating their opening and closing. Cell membranes are generally impermeable to ions, thus they must diffuse through the membrane through transmembrane protein channels. They have a crucial role in excitable cells such as neuronal and muscle tissues, allowing a rapid and co-ordinated depolarization in response to triggering voltage change. Found along the axon and at the synapse, voltage-gated ion channels directionally propagate electrical signals. Voltage-gated ion-channels are usually ion-specific, and channels specific to sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) ions have been identified. The opening and closing of the channels are triggered by changing ion concentration, and hence charge gradient, between the sides of the cell membrane.
Molecular neuroscience is a branch of neuroscience that observes concepts in molecular biology applied to the nervous systems of animals. The scope of this subject covers topics such as molecular neuroanatomy, mechanisms of molecular signaling in the nervous system, the effects of genetics and epigenetics on neuronal development, and the molecular basis for neuroplasticity and neurodegenerative diseases. As with molecular biology, molecular neuroscience is a relatively new field that is considerably dynamic.
Sodium channels are integral membrane proteins that form ion channels, conducting sodium ions (Na+) through a cell's membrane. They belong to the superfamily of cation channels.

Kv7.2 (KvLQT2) is a voltage- and lipid-gated potassium channel protein coded for by the gene KCNQ2.

Kv7.3 (KvLQT3) is a potassium channel protein coded for by the gene KCNQ3.

Neurofascin is a protein that in humans is encoded by the NFASC gene.

Potassium voltage-gated channel subfamily KQT member 4, also known as voltage-gated potassium channel subunit Kv7.4, is a protein that in humans is encoded by the KCNQ4 gene.

Potassium voltage-gated channel subfamily KQT member 5 is a protein that in humans is encoded by the KCNQ5 gene.

Sodium channel protein type 8 subunit alpha also known as Nav1.6 is a membrane protein encoded by the SCN8A gene. Nav1.6 is one sodium channel isoform and is the primary voltage-gated sodium channel at each node of Ranvier. The channels are highly concentrated in sensory and motor axons in the peripheral nervous system and cluster at the nodes in the central nervous system.

Ankyrin-3 (ANK-3), also known as ankyrin-G, is a protein from ankyrin family that in humans is encoded by the ANK3 gene.
M current is a type of noninactivating potassium current first discovered in bullfrog sympathetic ganglion cells.
Voltage-gated sodium channels (VGSCs), also known as voltage-dependent sodium channels (VDSCs), are a group of voltage-gated ion channels found in the membrane of excitable cells (e.g., muscle, glial cells, neurons, etc.) with a permeability to the sodium ion Na+. They are the main channels involved in action potential of excitable cells.

In neuroscience, ball and chain inactivation is a model to explain the fast inactivation mechanism of voltage-gated ion channels. The process is also called hinged-lid inactivation or N-type inactivation. A voltage-gated ion channel can be in three states: open, closed, or inactivated. The inactivated state is mainly achieved through fast inactivation, by which a channel transitions rapidly from an open to an inactivated state. The model proposes that the inactivated state, which is stable and non-conducting, is caused by the physical blockage of the pore. The blockage is caused by a "ball" of amino acids connected to the main protein by a string of residues on the cytoplasmic side of the membrane. The ball enters the open channel and binds to the hydrophobic inner vestibule within the channel. This blockage causes inactivation of the channel by stopping the flow of ions. This phenomenon has mainly been studied in potassium channels and sodium channels.
KCNQ2 encephalopathy typically presents with tonic seizures from the first week of life. The seizures can be frequent and often difficult to treat. Seizures can resolve within months or years but can impair the development of several domains such as motor, social, cognitive and language.














