Antimycins are produced as secondary metabolites by Streptomyces bacteria, a soil bacteria. These specialized metabolites likely function to kill neighboring organisms in order to provide the streptomyces bacteria with a competitive edge.
Nonribosomal peptides (NRP) are a class of peptide secondary metabolites, usually produced by microorganisms like bacteria and fungi. Nonribosomal peptides are also found in higher organisms, such as nudibranchs, but are thought to be made by bacteria inside these organisms. While there exist a wide range of peptides that are not synthesized by ribosomes, the term nonribosomal peptide typically refers to a very specific set of these as discussed in this article.

Viomycin is a member of the tuberactinomycin family, a group of nonribosomal peptide antibiotics exhibiting anti-tuberculosis activity. The tuberactinomycin family is an essential component in the drug cocktail currently used to fight infections of Mycobacterium tuberculosis. Viomycin was the first member of the tuberactinomycins to be isolated and identified, and was used to treat TB until it was replaced by the less toxic, but structurally related compound, capreomycin. The tuberactinomycins target bacterial ribosomes, binding RNA and disrupting bacterial protein synthesis and certain forms of RNA splicing. Viomycin is produced by the actinomycete Streptomyces puniceus.

Neocarzinostatin (NCS) is a macromolecular chromoprotein enediyne antitumor antibiotic secreted by Streptomyces macromomyceticus.
Polyketide synthases (PKSs) are a family of multi-domain enzymes or enzyme complexes that produce polyketides, a large class of secondary metabolites, in bacteria, fungi, plants, and a few animal lineages. The biosyntheses of polyketides share striking similarities with fatty acid biosynthesis.

Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway. Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of streptomyces. In contrast, only one known non-wild type species, streptomyces peucetius subspecies caesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of streptomyces can produce doxorubicin. His group has also cloned many of the genes required for DXR production, although not all of them have been fully characterized. In 1996, Strohl's group discovered, isolated and characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR. By 1999, they produced recombinant Dox A, a Cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin producing strains have the necessary genes to produce DXR, the much more therapeutically important of the two. Hutchinson's group went on to develop methods to improve the yield of DXR, from the fermentation process used in its commercial production, not only by introducing Dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed Dox A, were able to double the yield of DXR. This is of more than academic interest because at that time DXR cost about $1.37 million per kg and current production in 1999 was 225 kg per annum. More efficient production techniques have brought the price down to $1.1 million per kg for the non-liposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.
In enzymology, an erythronolide synthase is an enzyme that catalyzes the chemical reaction
Zwittermicin A is an antibiotic that has been identified from the bacterium Bacillus cereus UW85. It is a molecule of interest to agricultural industry because it has the potential to suppress plant disease due to its broad spectrum activity against certain gram positive and gram negative prokaryotic micro-organisms. The molecule is also of interest from a metabolic perspective because it represents a new structural class of antibiotic and suggests a crossover between polyketide and non-ribosomal peptide biosynthetic pathways. Zwittermicin A is linear aminopolyol.

Psymberin, also known as irciniastatin A, is a cytotoxin derived from sea sponges. It was discovered by two independent research groups, one led by Dr. Phil Crews and one led by Dr. Jean Schmidt, in 2004. Psymberin was found to be highly bioactive as it showed LC50s at nanomolar concentrations against various types of tumors.
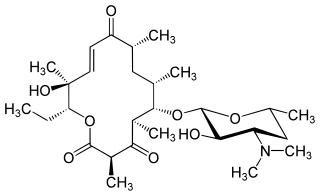
Pikromycin was studied by Brokmann and Hekel in 1951 and was the first antibiotic macrolide to be isolated. Pikromycin is synthesized through a type I polyketide synthase system in Streptomyces venezuelae, a species of Gram-positive bacterium in the genus Streptomyces. Pikromycin is derived from narbonolide, a 14-membered ring macrolide. Along with the narbonolide backbone, pikromycin includes a desosamine sugar and a hydroxyl group. Although Pikromycin is not a clinically useful antibiotic, it can be used as a raw material to synthesize antibiotic ketolide compounds such as ertythromycins and new epothilones.
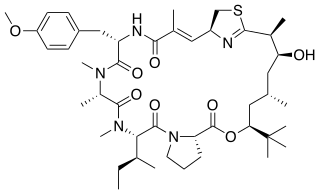
Apratoxin A - is a cyanobacterial secondary metabolite, known as a potent cytotoxic marine natural product. It is a derivative of the Apratoxin family of cytotoxins. The mixed peptide-polyketide natural product comes from a polyketide synthase/non-ribosomal peptide synthase pathway (PKS/NRPS). This cytotoxin is known for inducing G1-phase cell cycle arrest and apoptosis. This natural product's activity has made it a popular target for developing anticancer derivatives.
Fostriecin is a type I polyketide synthase (PKS) derived natural product, originally isolated from the soil bacterium Streptomyces pulveraceus. It belongs to a class of natural products which characteristically contain a phosphate ester, an α,β-unsaturated lactam and a conjugated linear diene or triene chain produced by Streptomyces. This class includes structurally related compounds cytostatin and phoslactomycin. Fostriecin is a known potent and selective inhibitor of protein serine/threonine phosphatases, as well as DNA topoisomerase II. Due to its activity against protein phosphatases PP2A and PP4 which play a vital role in cell growth, cell division, and signal transduction, fostriecin was looked into for its antitumor activity in vivo and showed in vitro activity against leukemia, lung cancer, breast cancer, and ovarian cancer. This activity is thought to be due to PP2A's assumed role in regulating apoptosis of cells by activating cytotoxic T-lymphocytes and natural killer cells involved in tumor surveillance, along with human immunodeficiency virus-1 (HIV-1) transcription and replication.

Borrelidin is an 18-membered polyketide macrolide derived from several Streptomyces species. First discovered in 1949 from Streptomyces rochei, Borrelidin shows antibacterial activity by acting as an inhibitor of threonyl-tRNA synthetase and features a nitrile moiety, a unique functionality in natural products., Borrelidin also exhibits potent angiogenesis inhibition, which was shown in a rat aorta matrix model. Other studies have been performed to show that low concentrations of borrelidin can suppress growth and induce apoptosis in malignant acute lymphoblastic leukemia cells. Borredlidin's antimalarial activity has also been shown in vitro and in vivo.
Butyrolactol A is an organic chemical compound of interest for its potential use as an antifungal antibiotic.
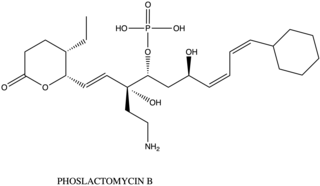
Phoslactomycin (PLM) is a natural product from the isolation of Streptomyces species. This is an inhibitor of the protein serine/threonine phosphatase which is the protein phosphate 2A (PP2A). The PP2A involves the growth factor of the cell such as to induce the formation of mitogen-activated protein interaction and playing a role in cell division and signal transduction. Therefore, PLM is used for the drug that prevents the tumor, cancer, or bacteria. There are nowsaday has 7 kinds of different PLM from PLM A to PLM G which differ the post-synthesis from the biosynthesis of PLM.
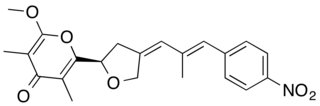
Aureothin is a natural product of a cytotoxic shikimate-polyketide antibiotic with the molecular formula C22H23NO6. Aureothin is produced by the bacterium Streptomyces thioluteus that illustrates antitumor, antifungal, and insecticidal activities and the new aureothin derivatives can be antifungal and antiproliferative. In addition, aureothin, a nitro compound from Streptomyces thioluteus, was indicated to have pesticidal activity against the bean weevil by interfering with mitochondrial respiratory complex II.

Prescopranone is a key intermediate in the biosynthesis of scopranones. Prescopranone is the precursor to scopranone A, scopranone B, and scopranone C, which are produced by Streptomyces sp. BYK-11038.
Andrimid is an antibiotic natural product that is produced by the marine bacterium Vibrio coralliilyticus. Andrimid is an inhibitor of fatty acid biosynthesis by blocking the carboxyl transfer reaction of acetyl-CoA carboxylase (ACC).

Peucemycin is a polyketide produced by Streptomyces peucetius, a Gram-positive filamentous bacteria that also produces the anticancer compounds daunorubicin and doxorubicin. This compound was elucidated from a cryptic biosynthetic gene cluster and is produced under temperature-specific conditions for bacterial growth. Peucemycin has demonstrated bioactivity against growth of S. aureus, P. hauseri, and S. enterica and also is weakly active against cancer cell lines. Peucemycin is biosynthesized through a Type 1 PKS system.

Disorazol, a cyclic polyketide synthesized by the bacterium Sorangium cellulosum So ce12, was first detected and isolated in 1994. Its chemical structure consists of a macrocyclic ring and two oxazole rings. Disorazol A has been demonstrated to exhibit anti-fungi activities, but it was not active against yeasts. In addition, this substance demonstrates potent anti-cancer characteristics at exceptionally low picomolar levels by obstructing the mechanism of tubulin assembly and triggering the disruption of microtubules. As a result, these impacts lead to the initiation of cell apoptosis. However, disorazols cannot be directly used as drugs in the clinic due to its extremely high cytotoxicity and instability. Thus, chemical and biosynthetic synthesis pathways were designed to synthesize unnatural derivatives of disorazol in hope of reducing its cytotoxicity without decreasing its anti-cancer potency.













